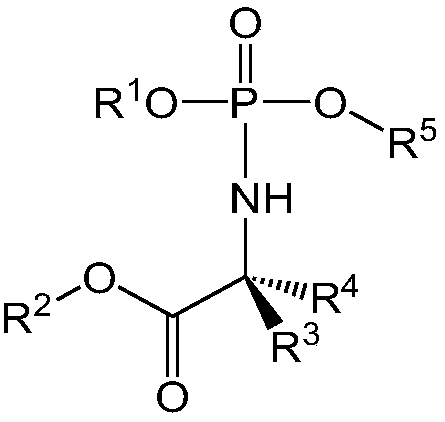
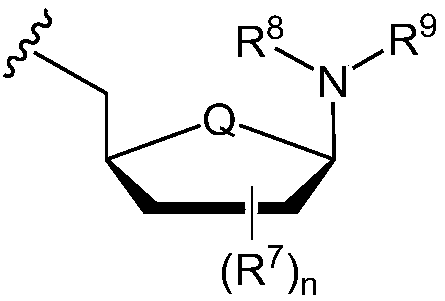
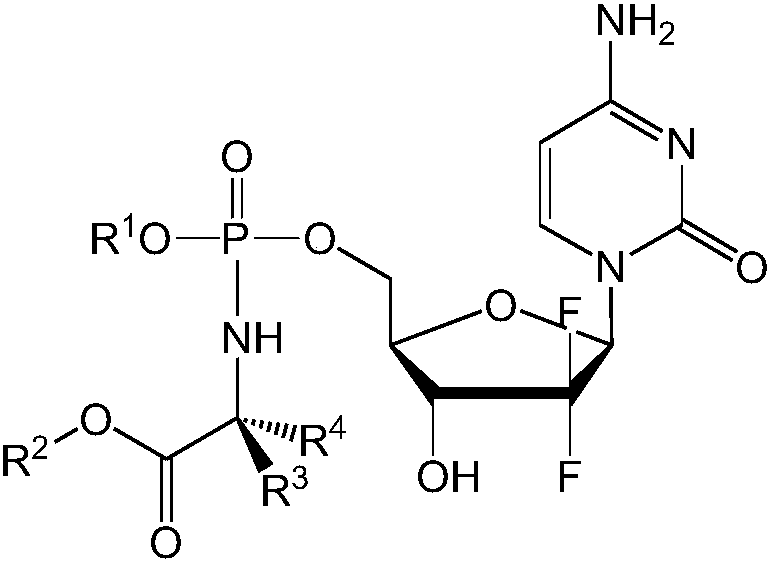
Formulations of phosphoramidate derivatives of nucleoside drugs
A technology of pharmaceutical preparations and phosphate esters, applied in the direction of drug combination, drug delivery, medical preparations of non-active ingredients, etc., can solve problems such as limited development and poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0283] Example 1 - Solubility of NUC-3373
[0284] Table 1 shows the solubility of NUC-3373 (a mixture of diastereomers) in a range of solvents.
[0285] Table 1: Solubility of NUC-3373 in a range of pharmaceutically relevant solvents
[0286]
[0287]
[0288] It can be easily seen that the solubility of NUC-3373 in water is extremely low. Among the solvents tested, polar aprotic solvents, especially DMSO and DMA provided the best solubility.
Embodiment 2
[0289] Example 2 - Development of an aqueous formulation of NUC-3373.
[0290] The successful development of the diluent solution enabled the preparation of an aqueous formulation of NUC-1031, which prompted its development for an aqueous formulation of NUC-3373. Aqueous formulations of NUC-3373 were developed by adding 6.7 ml of a 250 mg / ml NUC-3373 solution in 80% DMA:20% 0.9% saline to 10 ml of diluent solution to produce a 100 mg / ml NUC-3373 surfactant solution (see Table 4), then diluted into the infusion bag.
[0291] The clinical dose of NUC-3373 has not been determined, but the estimated maximum dose may be as much as 3,000 mg, which sets an upper limit for formulation development studies. Table 2 shows the volume of 100 mg / ml NUC-3373 surfactant solution that needs to be added to a 250 ml infusion bag for various doses, and the composition of the resulting aqueous infusion solution.
[0292] Table 2: Composition of saline infusion solutions with various doses of N...
Embodiment 3
[0310] Example 3 - Illustrative Description of Formulation Process
[0311] Formulation methodology (see WO2015 / 198059 (PCT / GB2015 / 051858)) has been developed for intravenous administration of phosphoramidate prodrugs. This approach has been shown in clinical trials to be effective against NUC-1031, which has approximately the same solubility profile as NUC-3373 and NUC-7738. The method is as follows: A 250 mg / mL solution of the phosphoramidite ester prodrug (S-epimer, R-epimer, or a mixture thereof) is formed in an 80:20 (by volume) mixture of DMA and 0.9% saline. This stock solution formulation is generally sufficiently stable for long-term storage and transport of the phosphoramidate prodrug.
[0312] The stock solution formulation can be administered intravenously to the patient by CVAD (eg Hickman series, PICC series). The intravenous administration set is typically flushed with an 80:20 (by volume) mixture of DMA and 0.9% saline before and after administration of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com