Stretchable self-repairing hydrogel based on dynamic covalent crosslinking agent and preparation method thereof
A covalent cross-linking and hydrogel technology, which is applied in the field of stretchable self-healing hydrogel and its preparation, can solve the problems of borate hydrogel self-healing performance and stretching performance to be improved, and achieve the goal of preparation Simple method, evenly distributed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Preparation of dynamic covalent crosslinking agent containing borate bond
[0042] (1) Preparation of AOI-2OH
[0043] The whole preparation reaction of AOI-2OH is at room temperature N 2 atmosphere, the specific steps are as follows:
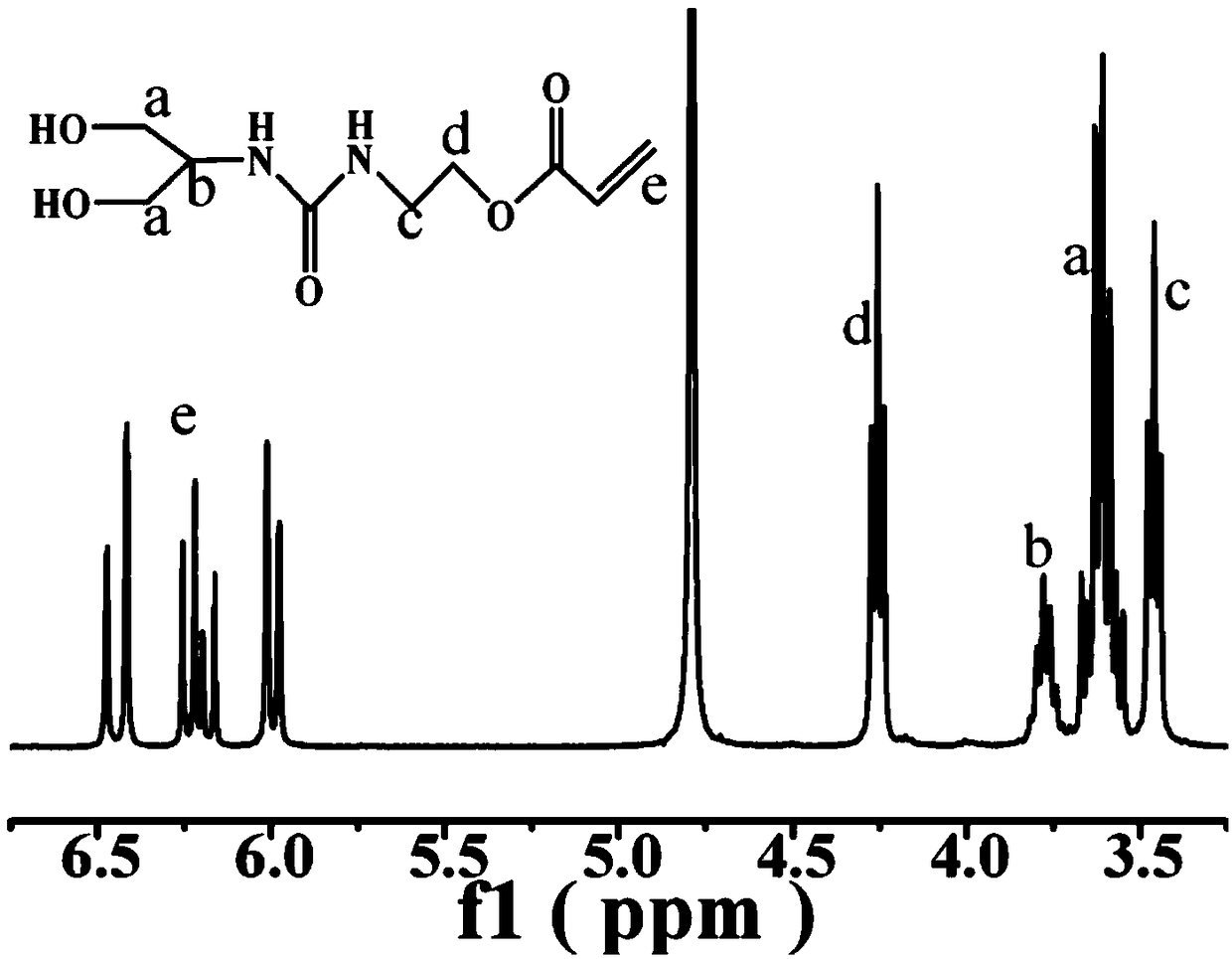
[0044] First, 1.37 g of 2-aminopropanediol (15 mmol) was dissolved in 3 mL of methanol, N 2 Stir in the atmosphere for 20 min, and add 2.11 g of AOI (15 mmol) after the solution is clear. After 16 hours, the reaction was terminated, the solvent was removed by filtration at room temperature, and the filtrate was dissolved and washed repeatedly with ethyl acetate for 4 to 5 times. Finally, the obtained white solid product was dried in a vacuum oven at 40°C for 12 hours to obtain a monomer containing a dihydroxyl group at the end, hereinafter referred to as AOI-2OH, and its synthesis route is as follows:
[0045]
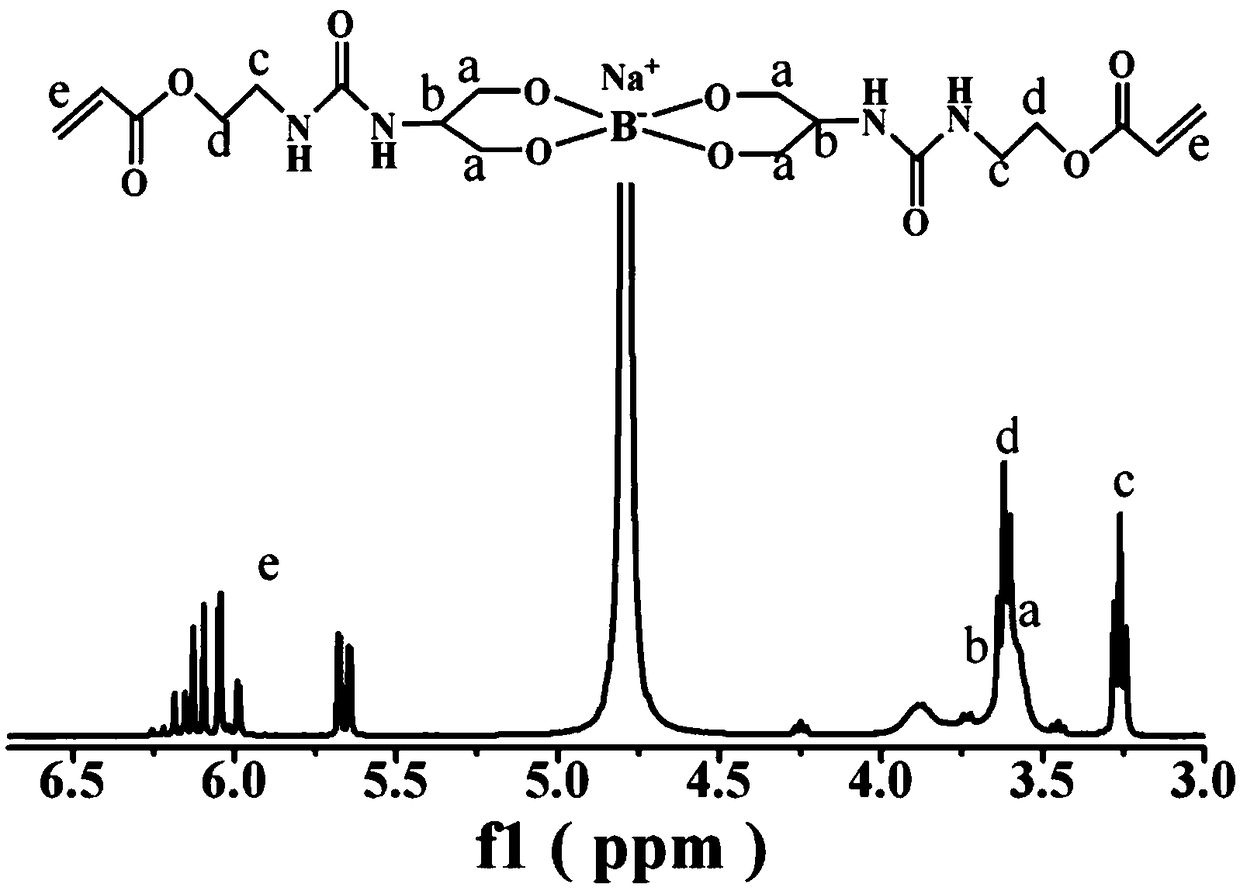
[0046] (2) Preparation of dynamic covalent crosslinker (DCL)
[0047] Add the monomer AOI-2OH synthesized ...
Embodiment 2
[0054] Embodiment 2 Preparation of self-healing hydrogel based on borate bond
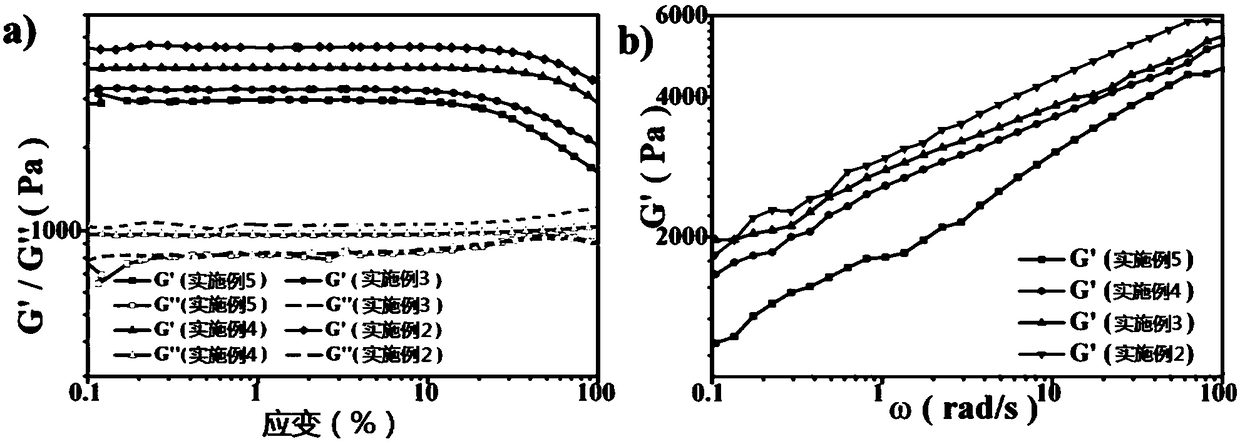
[0055] The DCL cross-linking agent prepared in Example 1 and acrylamide (hereinafter referred to as Am) are dissolved in water, and the molar ratio of the two is 1:4.5 to obtain an aqueous solution, and photoinitiator Irgacure 2959 is added thereto, the mass of acrylamide in the aqueous solution Fraction is 15%, photoinitiator dosage is 1.5% of Am mass fraction. After ultrasonically mixing the above substances, inject them into a disposable plastic syringe or a special mold, and polymerize under ultraviolet light for 6 hours to obtain a colorless and transparent hydrogel.
Embodiment 3
[0057] The hydrogel was prepared according to the method of Example 2, except that the molar ratio of DCL to Am was 1:5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fracture stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com