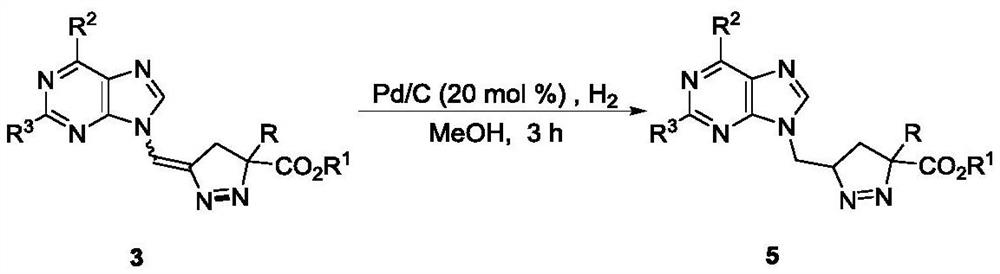
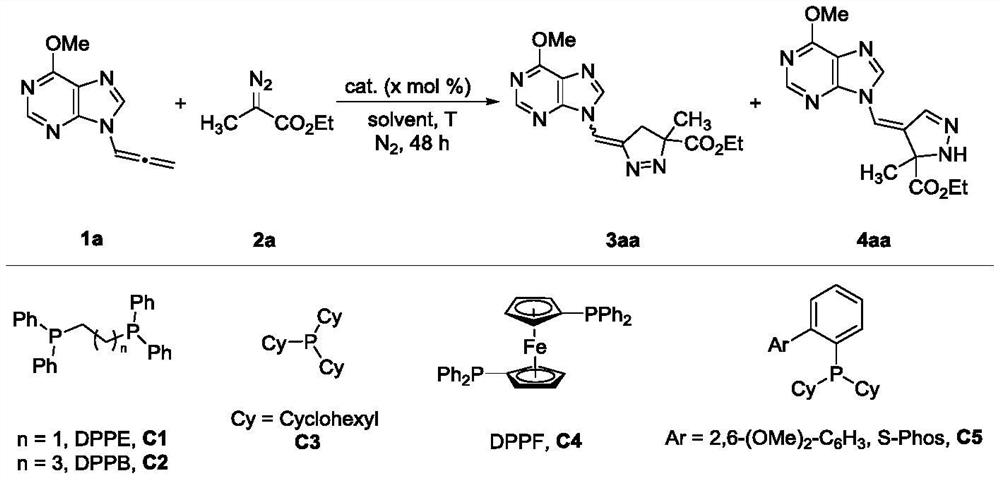
A kind of synthetic method of pyrazoline nucleoside analog with quaternary carbon center
A technology of pyrazoline nucleosides and synthetic methods, applied in the field of organic synthesis in organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027]
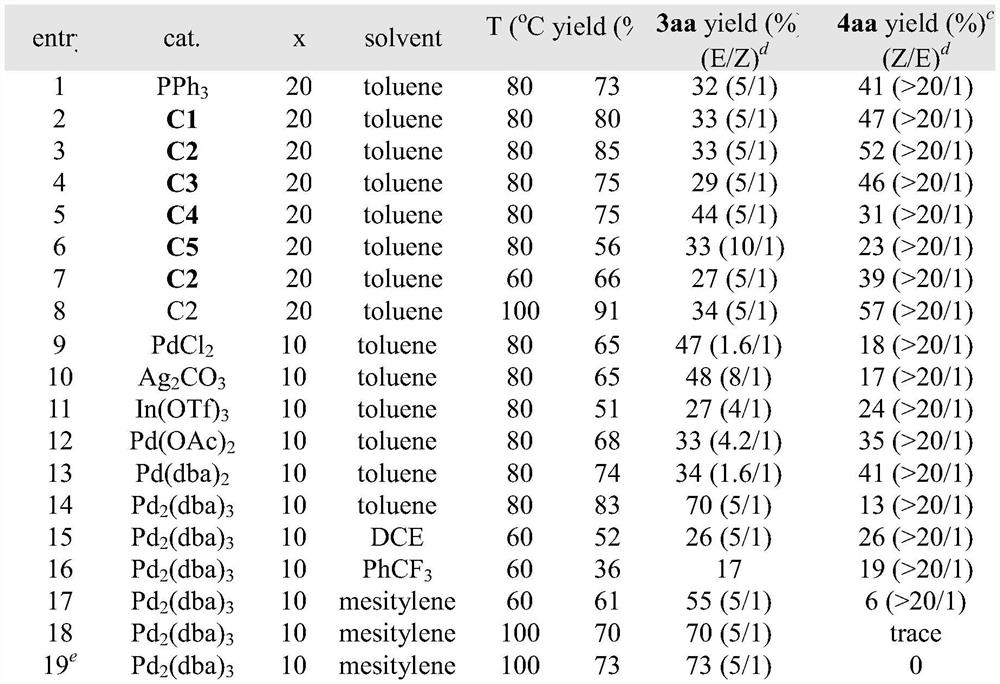
[0028] a Unless otherwise noted, reaction conditions were: cat.(20mol%),1a(0.05mmol),2a(0.3mmol)in solvent(1.0mL)under N 2 for 48h. b Total yield of 3aa and 4aa. c Isolated yield. d Determined by 1 H NMR spectroscopy of the crude reaction mixture. e 2a and Pd 2 (dba) 3 were added for two times in mesitylene.
[0029] In the screening process of reaction conditions, the influence of the catalyst on the reaction was first investigated (entries 1-6, 9-14), and DPPB was determined to be the best catalyst for obtaining various pyrazolines, Pd 2 (dba) 3 The best catalyst for obtaining a single 1-pyrazoline.
[0030] In the case of other conditions being fixed, only the influence of the solvent on the reaction was investigated (entries 15-17), and it was determined that toluene was the best solvent for obtaining various pyrazolines, and mesitylene was the best solvent for synthesizing 1-pyrazoline .
[0031] With other conditions fixed, only the inf...
Embodiment 2
[0033] Taking compounds 1a and 2a as an example, the reaction in Pd 2 (dba) 3 The reaction steps under catalysis are as follows:
[0034]
[0035] Weigh 9.4mg (0.05mmol, 1equiv) of compound 1a and put it in a clean 15mL reaction tube, then add 2.3mg of Pd 2 (dba) 3 (0.005 mmol, 5%) catalyst. The reaction tube was sealed, and the air in the reaction bottle was evacuated through a three-way tube, and then nitrogen gas was introduced, and 19.2 mg of compound 2a (0.15 mmol, 3 equiv) was weighed and dissolved in 1 mL of mesitylene solution, and added to the reaction with a syringe , and the reaction was completely carried out under nitrogen conditions at 100°C. After 24 hours of reaction, inject 2.3 mg of Pd again 2 (dba) 3 (0.005mmol, 5%) catalyst, 19.2mg of compound 2a (0.15mmol, 3equiv) in mesitylene solvent, continue to react for 24 hours. Detect with TCL and find that raw material disappears substantially. The reaction solution was taken out, and the solvent in the ...
Embodiment 3
[0041] Taking 1a and 2a compounds as an example, the reaction steps of this reaction under the catalysis of bis(diphenylphosphine)butane DPPB are as follows:
[0042]
[0043] Weigh 18.8 mg (0.1 mmol, 1 equiv) of compound 1a into a clean 20 mL reaction tube, then add 8.5 mg of DPPB (0.02 mmol, 20%) catalyst. The reaction tube was sealed, and the air in the reaction flask was evacuated through a three-way tube, and then nitrogen gas was introduced, and 38.4 mg of compound 2a (0.3 mmol, 3 equiv) was weighed and dissolved in 1.5 mL of toluene solution, and added to the reaction with a syringe. And make the reaction completely under nitrogen condition, 100 degrees centigrade, carry out. After 48 hours of reaction, it was detected by TCL that the raw materials basically disappeared. The reaction solution was taken out, and the solvent in the solution was spin-dried with a vacuum rotary evaporator, and column chromatography was used to separate the solvent in a 3:1 development s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com