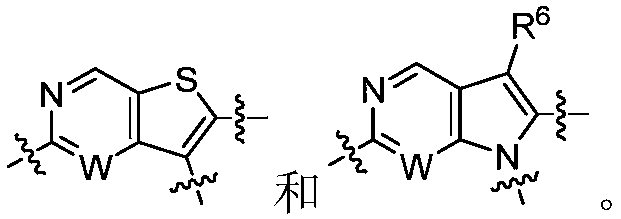
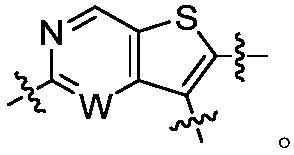
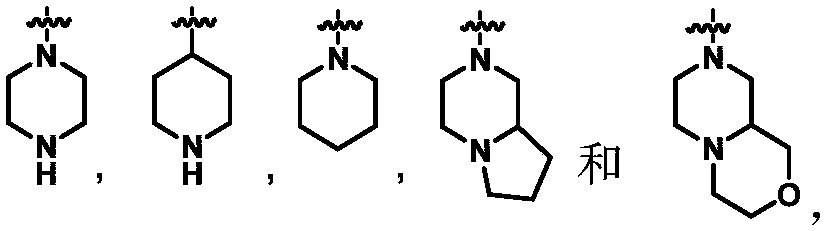
Certain protein kinase inhibitors
A compound and alkyl technology, applied in the field of treatment of hyperproliferative diseases, can solve the problems of low inhibitor activity, toxicity, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0400] 1-cyclopentyl-N,N-dimethyl-6-((5-(piperazin-1-yl)pyrimidin-2-yl)amino)-1H-pyrrole[3,2-c]pyrrole Pyridine-2-carboxamide (1)
[0401]
[0402] tert-Butyl 4-(2-aminopyrimidin-5-yl)piperazine-1-carboxylate (1a)
[0403] 2-Amino-5-bromopyrimidine (3.5g, 20.1mmol), N-Boc-piperazine (8.2g, 44.1mmol), sodium tert-butoxide (3.3g, 34.4mmol), three (dibenzylidene acetone ) Dipalladium 0.92g, 1.0mmol) and 2-di-tert-butylphosphine-2'-(N,N-dimethylamino)biphenyl (1.37g, 4.0mmol) were added in toluene (150mL) and mixed and stirred, Heated to 110°C under protection to react overnight. The reaction system was cooled to room temperature and filtered with Celite on a Buchner funnel, and the filtrate was concentrated. The residue was dissolved with 0.5N hydrochloric acid (200 mL) and extracted with ethyl acetate (2×100 mL). The aqueous phase was basified with sodium hydroxide, adjusted to pH 4 and extracted with dichloromethane (3 x 150 mL). The combined organic layers were dr...
Embodiment 2
[0409] 1-cyclopentyl-N,N-dimethyl-6-((5-(4-methylpiperazin-1-yl)pyrimidin-2-yl)amino)-1H-pyrrole[3, 2-c]pyridine-2-carboxamide (2)
[0410]
[0411] To 1-cyclopentyl-N,N-dimethyl-6-((5(piperazin-1-yl)pyrimidin-2-yl)amino)-1H-pyrrole[3,2-c at 0°C ]Pyridine-2-carboxamide (1) (11 mg, 0.025 mmol) in 1,2-dichloroethane (3 mL) was added formaldehyde (37% aqueous solution, 10 μ L), sodium triacetoxyborohydride (54 mg, 0.25mmol), and then reacted at this temperature for 1 hour. The reaction mixture was diluted with saturated sodium bicarbonate solution (10 mL) and extracted with dichloromethane (2 x 30 mL). Dry over anhydrous sodium sulfate and concentrate. The residue was purified by preparative thin-layer chromatography (developing solvent: dichloromethane / methanol 15:1) to give 1-cyclopentyl-N,N-dimethyl-6-((5-(4-methylpiperazine -1-yl)pyrimidin-2-yl)amino)-1H-pyrrolo[3,2-c]pyridine-2-carboxamide (2). MS-ESI(m / z):449[M+1] + .
Embodiment 3
[0413] 1-cyclopentyl-6-((5-(4-ethylpiperazin-1-yl)pyrimidin-2-yl)amino)-N,N-dimethyl-1H-pyrrole[3, 2-c]pyridine-2-carboxamide (3)
[0414]
[0415]To 1-cyclopentyl-N,N-dimethyl-6-((5-(piperazin-1-yl)pyrimidin-2-yl)amino)-1H-pyrrole[3,2- c] To a solution of pyridine-2-carboxamide (1) (8.2mg, 0.019mmol) in 1,2-dichloroethane (3mL) was added acetaldehyde (40% aqueous solution, 10μL), sodium triacetoxyborohydride (40mg, 0.19mmol), and then reacted at this temperature for 1 hour. The reaction mixture was diluted with saturated sodium bicarbonate solution (10 mL) and extracted with dichloromethane (2×30 mL). Dry over anhydrous sodium sulfate and concentrate. The residue was purified by preparative thin-layer chromatography (developing solvent: dichloromethane / methanol 15:1) to give 1-cyclopentyl-6-((5-(4-ethylpiperazin-1-yl)pyrimidine-2 -yl)amino)-N,N-dimethyl-1H-pyrrolo[3,2-c]pyridine-2-carboxamide (3). MS-ESI(m / z):463[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com