Chimeric antigen receptors targeting her2
A chimeric antigen receptor, targeting sequence technology, applied in the direction of antibodies, targeting specific cell fusion, peptides containing localization/targeting motifs, etc., can solve the problem of low tumor specificity of T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
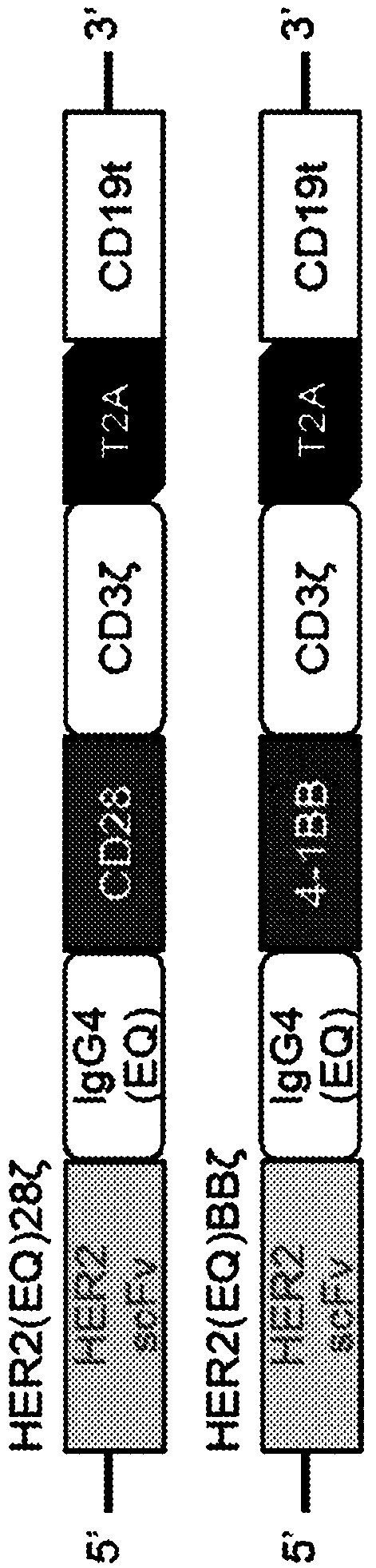
[0049] Example 1: Structures of two HER2-CARs
[0050] One CAR comprising the HER2 scFv described herein is called Her2scFv-IgG4(L235E, N297Q)-CD28tm-CD28gg-Zeta-T2A-CD19t. This CAR includes several important features including: scFv targeting HER2; IgG4 Fc region mutated (L235E; N297Q) at two sites within the CH2 region in a manner that reduces Fc receptor (FcR) binding; CD28 spanning Membrane domain, CD28 co-stimulatory domain and CD3ζ activation domain. figure 1 The amino acid sequence of this CAR is presented, including the sequence of the truncated CD19 sequence used to monitor CAR expression and the T2A ribosomal skipping sequence that allows CAR production without fusion of the truncated CD19 sequence. like figure 2 As shown, the immature CAR includes: GMCSFR signal peptide, HER2 scFv, IgG4 acting as a spacer, CD8 transmembrane region, 4-1BB co-stimulatory domain containing sequence changes from LL to GG, triple Gly sequence, CD3 Zeta stimulatory domain . The tra...
Embodiment 2
[0051] Example 2: Construction and structure of epHIV7 for expressing HER2-specific CAR T cells
[0052] The epHIV7 vector is a vector that can be used to express HER2-specific CAR. It was generated from the pHIV7 vector. Importantly, this vector uses the human EFI promoter to drive CAR expression. Both the 5' and 3' sequences of the vector were derived from the pv653 RSN, as previously derived from the HXBc2 provirus. The polypurine tract DNA flap sequence (cPPT) was derived from the HIV-I strain pNL4-3 in the NIH AIDS Reagent Repository. Woodchuck post-transcriptional regulatory element (WPRE) sequences were described previously.
[0053]Construction of pHIV7 was performed as follows. Briefly, a pv653 RSN containing 653bp from gag-pol plus 5' and 3' long terminal repeats (LTRs) and an intervening SL3-neomycin phosphotransferase gene (Neo) was subcloned into pBluescript as follows: In step 1, p5'HIV-I 5I was prepared from the 5'LTR to the sequence of the rev-response e...
Embodiment 3
[0059] Example 3: Preparation of Vectors for Transducing Patient T Cells
[0060] Vectors for transduction of patient T cells can be prepared as follows. For each plasmid expressing the CAR, expressing the CAR and optionally a marker such as truncated CD19; 2) pCgp; 3) pCMV-G; and 4) pCMV-Rev2), a seed bank was generated which was used to inoculate the fermentation tank to produce a sufficient amount of plasmid DNA. Plasmid DNA was tested for identity, sterility, and endotoxin before being used to generate lentiviral vectors.
[0061] Briefly, cells were expanded from 293T working cells (WCB) that were tested to be sterile and free of viral contamination. Thaw a vial of 293T cells from 293T WCB. Cells were grown and expanded until sufficient numbers of cells were present to plate the appropriate number of 10-layer cell factories (CFs) for vector production and cell culture maintenance. Single trains of cells can be used for production.
[0062] Generate lentiviral vecto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com