Bisoxazoline ligand compound based on tetramethyl spirobiindane skeleton and intermediate, preparation method and application of bisoxazoline ligand compound
A technology of tetramethylspirodihydroindene and bisoxazoline, which is applied in the field of bisoxazoline ligand compounds, can solve the problems of lengthy reaction steps, high preparation cost, affect practicability and the like, and achieves easy large-scale application, The effect of a simple synthetic reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
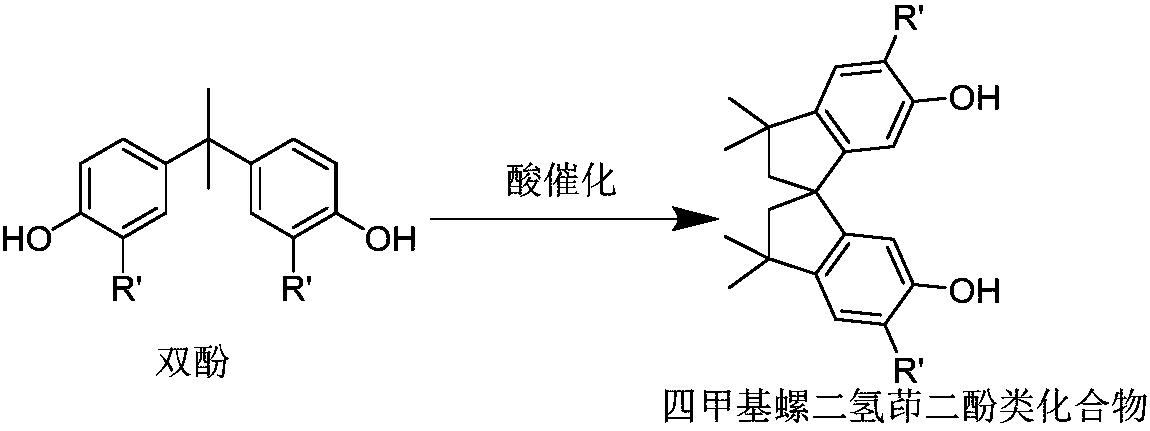
Image
Examples
Embodiment 1
[0036] 3,3,5,3',3',5'-hexamethyl-1,1'-spiroindane-7,7'-dicarbaldehyde (II-b) and 3,3,5,3' ,Synthesis of 3',5'-hexamethyl-1,1'-spiroindane-7,7'-dicarboxylic acid (III-b)
[0037]
[0038] The first step: In a 500mL three-necked flask, add 3,3,5,3',3',5'-hexamethyl-1,1'-spirodihydroindane-6,6'-diol (HMSPINOL, 10mmol, 3.4g), hexamethylenetetramine (HMTA, 80mmol, 11.2g), N 2 Protect. Then 120 mL of trifluoroacetic acid was added, and the reaction was refluxed overnight. The next day, 120 mL of glacial acetic acid was added, and the reflux reaction was continued for 3 days. Then the temperature was lowered to 95° C., 120 mL of hydrochloric acid (4 mol / L) was added, stirred for 5 hours, the reaction was stopped, and the temperature was lowered to room temperature. The reaction solution was poured into water, a large amount of precipitates were precipitated, and the yellow solid product II-b1 (3.2 g, yield: 82%) was obtained by suction filtration. m.p.283.7-285.6°C; 1 H NMR ...
Embodiment 2
[0043] (S)-3,3,5,3',3',5'-hexamethyl-1,1'-spiroindane-7,7'-dicarbaldehyde ((S)-II-b) and (S)-3,3,5,3',3',5'-Hexamethyl-1,1'-spiroindane-7,7'-dicarboxylic acid ((S)-III-b) synthesis
[0044]According to the process of Example 1, chiral (S)-3,3,5,3',3',5'-hexamethyl-1,1'-spirodihydroindene-6,6'-bis alcohol ((S)-HMSPINOL) instead of 3,3,5,3',3',5'-hexamethyl-1,1'-spiroindane-6,6'-diol (HMSPINOL), i.e. The corresponding (S)-3,3,5,3',3',5'-hexamethyl-1,1'-spirodihydroindene-7,7'-dicarbaldehyde ((S)- II-b, total yield 65%) and (S)-3,3,5,3',3',5'-hexamethyl-1,1'-spiroindane-7,7'-bis Formic acid ((S)-III-b, 60% overall yield).
Embodiment 3
[0046] Synthesis of 3,3,3',3'-tetramethyl-1,1'-spiroindane-6,6'-dihydroxy-7,7'-dicarbaldehyde (II-c)
[0047]
[0048] Add 5.2g compound (BMSPINOL, 12.4mmol), 10.8g hexamethylenetetramine (HMTA, 77mmol) in the reaction bottle, nitrogen protection, add 150mL trifluoroacetic acid (TFA), heat and reflux overnight, then add 150mL acetic acid , continue the reflux reaction for 72 hours, add 200 mL of 6 mol / L hydrochloric acid, stir and reflux for 28 hours for hydrolysis, add 100 mL of water, continue stirring and reacting for 24 hours, then cool the reaction solution, suction filter and fully wash the filter cake with water, and dry the filter cake to obtain 4.6g yellow powdery solid product II-aa, yield 80%. The melting point is 226-227°C. 1 H NMR (400MHz, CDCl 3 )δ12.55(s, 2H), 9.60(s, 2H), 7.30(s, 2H), 2.56(d, J=13.5Hz, 2H), 2.39(d, J=13.5Hz, 2H), 1.42( s,18H),1.37(s,6H),1.35(s,6H).
[0049] Add 2g of compound II-aa (4.2mmol) and 10g of anhydrous aluminum chloride (75mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com