Method of extracting lithium chloride from sulfate-type salt lake brine
A kind of salt lake brine and sulfate type technology, applied in the direction of lithium halide, etc., can solve the problem of high price of extraction agent, and achieve the effect of simple reaction route, high reaction yield and high industrial production value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The extractant is TBP and amide compound N,N-bis(2-ethylhexyl)acrylamide, whose structural formula is as follows:
[0022]
[0023] Its synthesis method is as follows: Add N,N-di-(2-ethylhexyl)amine into a three-necked flask, then add 1.1 equivalents of triethylamine, add dichloromethane as a solvent, and drop the system temperature to -5 ℃~0℃, dropwise add acryloyl chloride / dichloromethane solution, control the system temperature at 0℃, react for 2h, add water solution, collect the organic phase, and spin dry to obtain the target product.
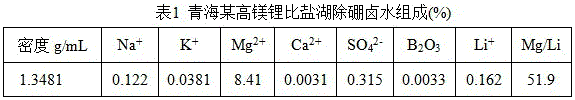
[0024] Add 1 volume of salt lake brine shown in Table 1 into a separatory funnel, add a certain amount of FeCl 3 (FeCl 3 Li in brine + The molar ratio is 1:1.3) as a co-extraction agent, shake to dissolve it. Add 2 volumes of organic phase (compared to O / A=2), where the volume ratio of N,N-di(2-ethylhexyl)acrylamide, TBP and 200# solvent naphtha is 3:2:5, shake for 5 After 10 minutes rest layered. Determination of Li in the ...
Embodiment 2
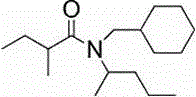
[0028] The extractant is TBP and amide compound N-(1-methylbutyl)-N-cyclohexamethylene-3-methyl-2-butenamide, and its structural formula is as follows:
[0029]
[0030] Add 1 volume of salt lake brine shown in Table 1 into a separatory funnel, add a certain amount of FeCl 3 (FeCl 3 Li in brine + The molar ratio is 1:1.5) as a co-extraction agent, shake to dissolve it. Add 5 volumes of organic phase (compared to O / A = 5) with N-(1-methylbutyl)-N-cyclohexamethylene-3-methyl-2-butenamide, TBP and sulfo The volume ratio of kerosene is 2:1:2, and after shaking for 10 minutes, the layers are still separated. Determination of Li in the equilibrium aqueous phase + concentration, the single extraction rate of lithium was calculated to be 88.01%, and the yield reached 98.67% after three countercurrent extractions.
[0031] Comparison results: The amide compound extractant shown in the following structural formula was synthesized. Using the same method as above, the yield reache...
Embodiment 3
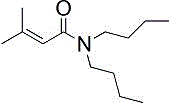
[0034] The extractant is TBP and amide compound N,N-dibutyl-2-methyl-2-butenamide, and its structural formula is as follows:
[0035]
[0036] Add 1 volume of salt lake brine shown in Table 1 into a separatory funnel, add a certain amount of FeCl 3 (FeCl 3 Li in brine + The molar ratio is 1:1) as a co-extraction agent, shake to dissolve it. Add 2 volumes of organic phase (compared to O / A = 0.8) with N,N-dibutyl-2-methyl-2-butenamide, TBP and sulfonated kerosene in a volume ratio of 1:1:2 , shake for 10 minutes and then rest for stratification. Determination of Li in the equilibrium aqueous phase + concentration, the calculated lithium extraction rate was 82.15%, and the yield reached 96.70% after three countercurrent extractions.
[0037] Comparison results: The amide compound extractant shown in the following structural formula was synthesized. Using the same method as above, the yield reached 81.28% after three countercurrent extractions.
[0038]
PUM
| Property | Measurement | Unit |
|---|---|---|
| extraction efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com