Application of rotenone in preparation of medicine for treating systemic lupus erythematosus
A systemic technology for lupus erythematosus, applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve problems such as disordered mitochondrial function, damaged protein nucleic acid, increased ROS, etc., to inhibit the formation of lymph node tumors, inhibit abnormal activation, and inhibit Effects of an enlarged spleen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
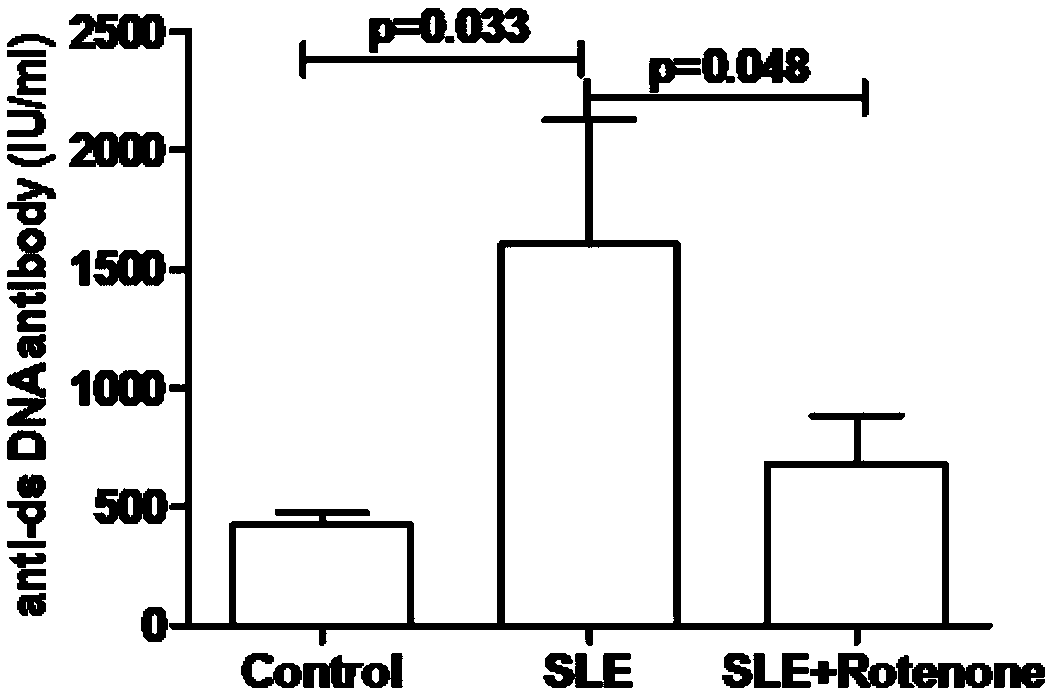
[0021] Example 1 Rotenone inhibits the production of antinuclear antibodies in the serum of MRL / Ipr lupus mice
[0022] The mice in the model group, drug-dosed group, and control group that were fed to the 33rd week were taken separately, and 8 mice were taken from each group. After the mice were anesthetized, blood was taken from the inferior vena cava, placed in an anticoagulant tube, and allowed to stand at room temperature for 30 minutes. Minutes later, the serum was centrifuged and the anti-dsDNA-antibody kit was used to detect the concentration of antinuclear antibody in the serum. For specific operation steps, refer to the kit instruction manual. The result is as figure 1 As shown, the average concentration of antinuclear antibody in the mouse serum of the control group, the model group and the drug-added group were 424IU / mL, 1602IU / mL and 681IU / mL, respectively, and the concentration of antinuclear antibody in the mouse serum of the model group was significantly higher...
Embodiment 2
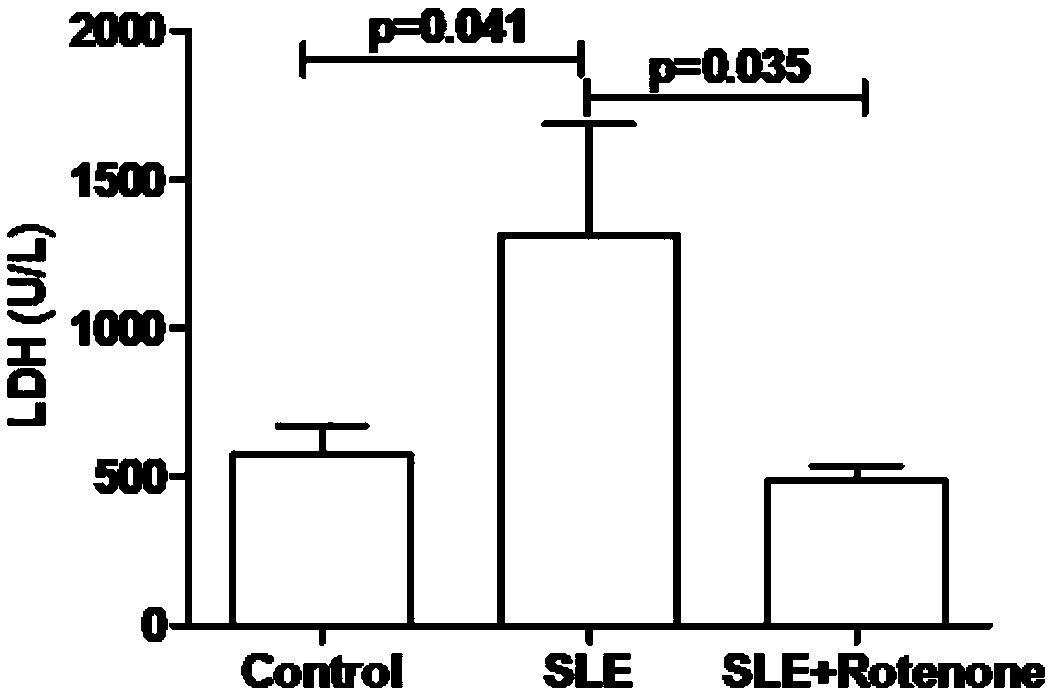
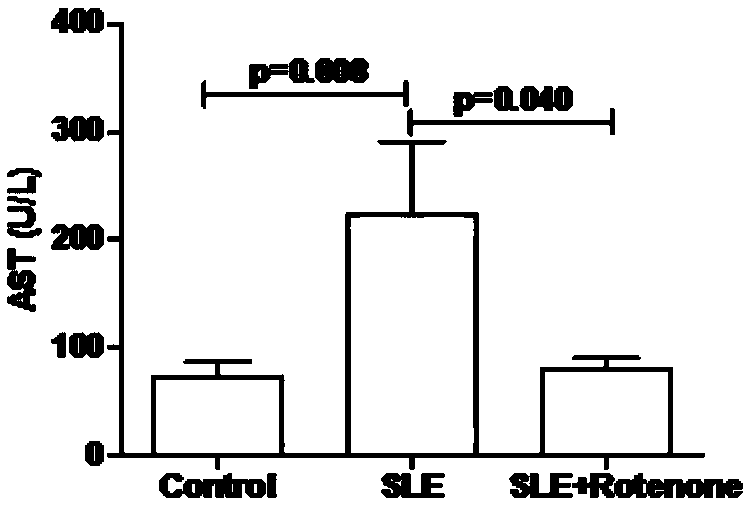
[0023] Example 2 Rotenone alleviates myocardial function damage in MRL / Ipr lupus mice
[0024] The mice in the model group, drug-dosed group, and control group that were fed to the 33rd week were taken separately, and 8 mice were taken from each group. After the mice were anesthetized, blood was taken from the inferior vena cava, placed in an anticoagulant tube, and allowed to stand at room temperature for 30 minutes. Minutes later, the serum was centrifuged, and the concentrations of lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) in the serum were detected by a biochemical analyzer, and the results were as follows: figure 2 and image 3 As shown, the average concentration of LDH in the mouse serum of the control group, the model group and the drug-added group was 577U / L, 1312U / L and 487U / L respectively, and the average concentration of AST in the mouse serum of the control group, the model group and the drug-added group Concentrations were 73U / L, 223U / L an...
Embodiment 3
[0025] Example 3 Rotenone inhibits abnormal activation of spleen B lymphocytes and splenomegaly in MRL / Ipr lupus mice
[0026] The mice in the model group, drug-dosed group, and control group that were fed to the 33rd week were taken respectively, 5 mice were taken from each group, the spleens of the mice were separated, and the size of the spleens was observed, as shown in Fig. Figure 4 As mentioned above, the spleens of the mice in the dosing group were significantly smaller than those in the model group. The spleen was ground and passed through a 200-mesh sieve, washed with pre-cooled PBS to obtain a single cell suspension, and the erythrocytes were lysed with erythrocyte lysate, stained with different fluorescently labeled B220 antibodies and CD69 antibodies, and incubated at 4°C in the dark for 30 minutes. After washing with PBS for 3 times, the number of B220 and CD69 double-positive cells and the ratio of B220 and CD69 double-positive cells to all spleen cells were det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com