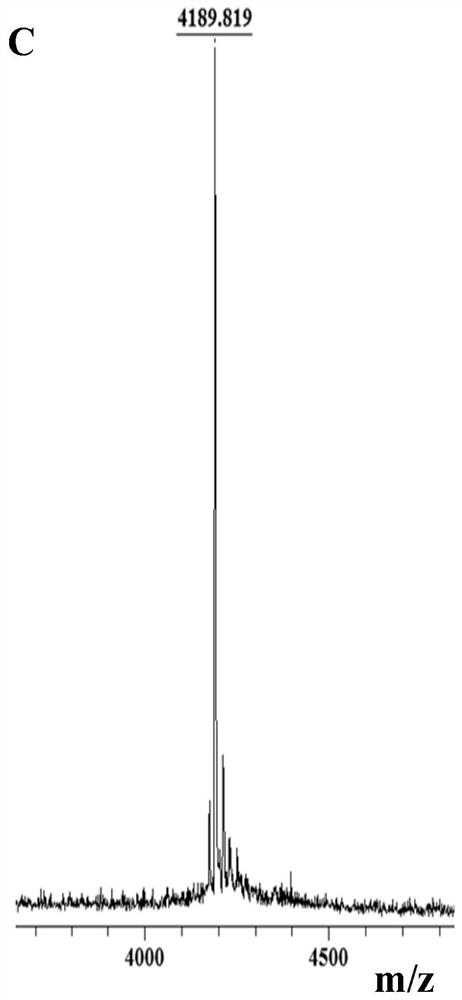Exenatide analogs with hydroxylamine groups and applications thereof
A technology of exenatide and analogs, applied in the field of medicinal chemistry, can solve problems such as unreported drug properties, and achieve the effects of facilitating optimization and development, reducing rapid filtration and prolonging half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
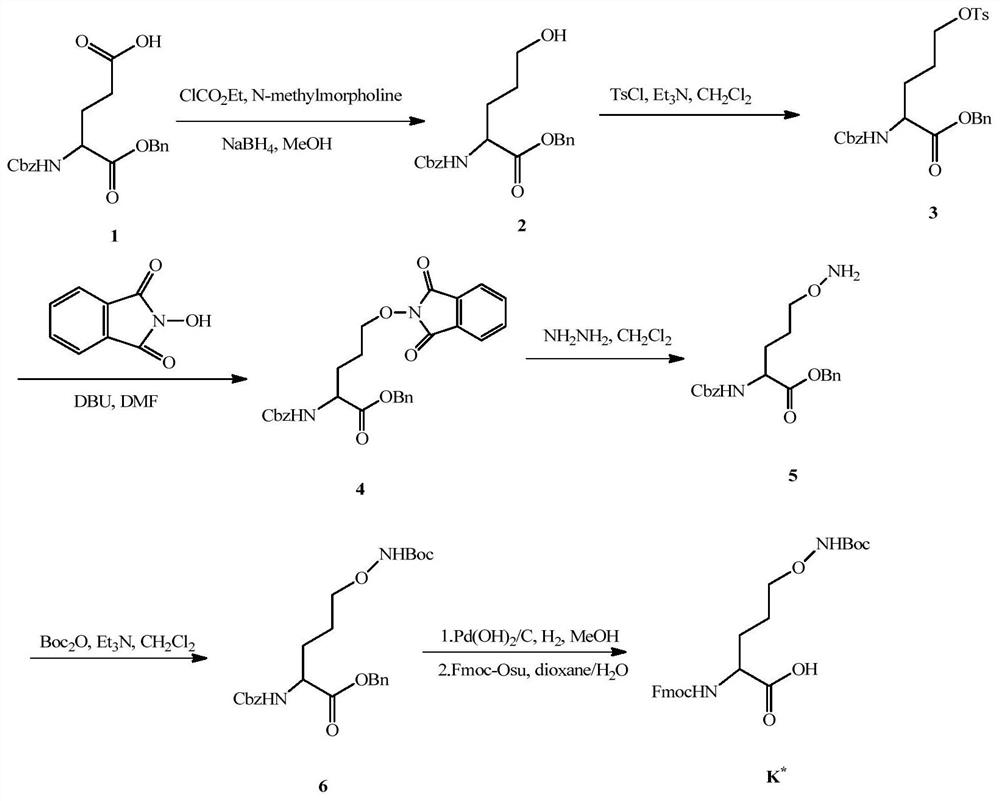
[0037] 1.1 Lysine analog K with hydroxylamine side chain * Synthesis
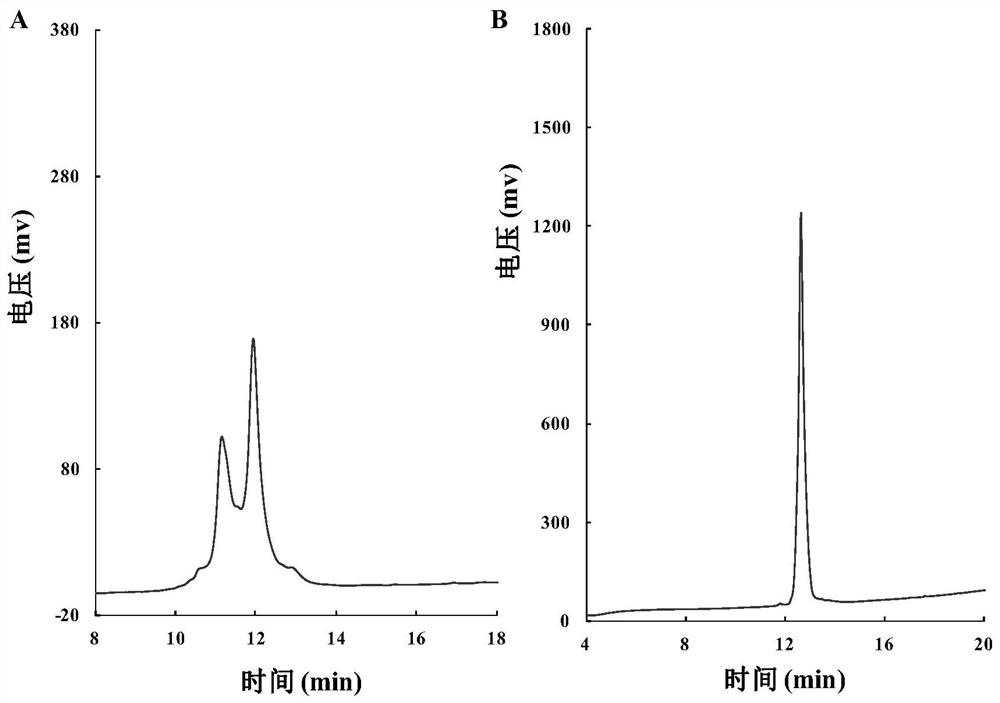
[0038] like figure 1 As shown, Z-Glu-Obzl (1-benzyl-N-benzyloxycarbonyl-L-glutamic acid) was used as a synthetic lysine analog K * The starting material of , obtain the lysine analogue K containing hydroxylamine side chain through 7 steps reaction * , the total yield was 32.1%. The Fmoc-protected lysine analog K * It can be directly used in solid-phase synthesis of polypeptides.
[0039] The starting material was commercial Z-Glu-OBzl, and Z-Glu-Obzl (22.3 g, 60 mmol) was dissolved in dry THF (150 mL). Add sodium chloride into the ice to make ice salt, adjust the temperature of the ice salt by controlling the amount of sodium chloride added, and adjust the temperature to about -10°C. Place the Z-Glu-OBzl solution on an ice-salt bath at -10°C and stir, then cool and stir for 10 min, and pay attention to prevent water from entering the solution. Add N-methylmorpholine (N-methylmorpholine, 6.6mL, 60mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com