A kind of polyβ-pinene resin functional modification method
A pinene resin and functionalization technology, which is applied in the field of functional modification of poly-β-pinene resin, can solve the problems of inability to realize functional modification of β-pinene resin and low insertion rate of β-pinene, and achieve Improve the original function, high selectivity, easy separation and purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 mercaptopropionic acid modified β-pinene resin
[0039] 1. Method
[0040] (1) In the 20mL dry amp tube, add 1.088g poly-β-pinene resin (industrial product, number average molecular weight is 6635), 0.102g benzoin dimethyl ether, 2.544g mercaptopropionic acid and 8mL toluene as solvent successively, Nitrogen deoxygenation, tube sealing.
[0041] (2) After the reaction was continued for 12 hours under the irradiation of a UV lamp with a wavelength of 365nm, the reaction solution was added dropwise into 40mL of methanol to precipitate the product, filtered and dried in vacuum at 40°C to obtain mercaptopropionic acid-modified β-pinene resin.
[0042] 2. Results
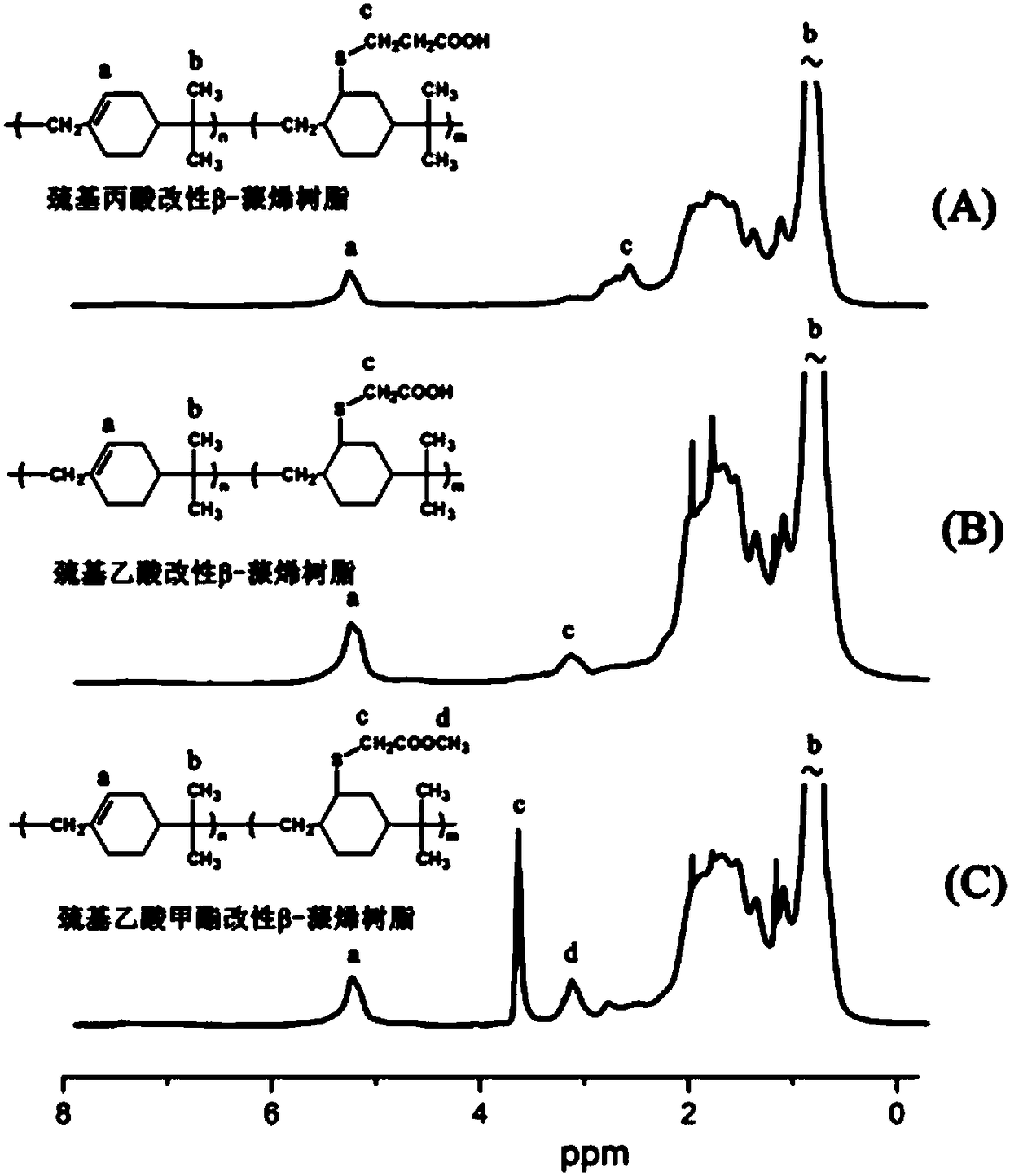
[0043] Mercaptopropionic acid modified poly-β-pinene resin 1 See attached for H NMR spectrum figure 1 As shown in Figure A in the middle, the grafting rate of mercaptopropionic acid measured by the peak area of the characteristic peak is 27%, that is, 27% of the monomer units on the poly-β-pinen...
Embodiment 2
[0045] Embodiment 2 mercaptoacetic acid modified β-pinene resin
[0046] 1, by the same synthetic method of embodiment 1, but feed intake is changed into 1.088g poly-pinene resin, 0.102g benzoin dimethyl ether and 2.208g thioglycolic acid. After treatment under the same conditions, thioglycolic acid modified β-pinene resin was obtained.
[0047] 2. Thioglycolic acid modified β-pinene resin 1 See attached for H NMR spectrum figure 1 As shown in Figure B, the grafting rate of thioglycolic acid measured by the peak area of the characteristic peak is 15%.
[0048] as attached image 3 As shown, thioglycolic acid modified β-pinene resin (modified product) can self-emulsify in water to form nanoparticles (which is expected to be used in drug delivery system), and its particle size is 223nm as measured by dynamic light scattering. The morphology is spherical micelles.
Embodiment 3
[0049] Embodiment 3 methyl mercaptoacetate modified β-pinene resin
[0050] 1, according to the same synthetic method of embodiment 1, but feed intake is changed into 1.088g poly-β-pinene resin, 0.090g 2-hydroxyl-4'-(2-hydroxyethoxy)-2-methyl propiophenone and 2.544 g methyl thioglycolate. Post-treatment under the same conditions to obtain methyl thioglycolate modified β-pinene resin.
[0051] 2. Methyl thioglycolate modified β-pinene resin 1 See attached for H NMR spectrum figure 1 As shown in Figure C in the middle, the grafting rate of methyl thioglycolate is 21% measured by the peak area of the characteristic peak.
[0052] 3. In addition, the above-mentioned methyl thioglycolate-modified β-pinene resin and acrylic resin (industrial product, glass transition temperature 15° C.) were blended in an equal mass ratio by using a solution blending method.
[0053] The DSC test shows that the glass transition temperature of the modified β-pinene resin component in the blend i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com