Method for synthesizing kaliophilite fluorescent material
A technology of fluorescent materials and synthesis methods, which is applied in the field of synthesis of kalephine fluorescent materials, can solve problems such as high production costs and complex synthesis processes, and achieve the effects of low cost, simple synthesis procedures, and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
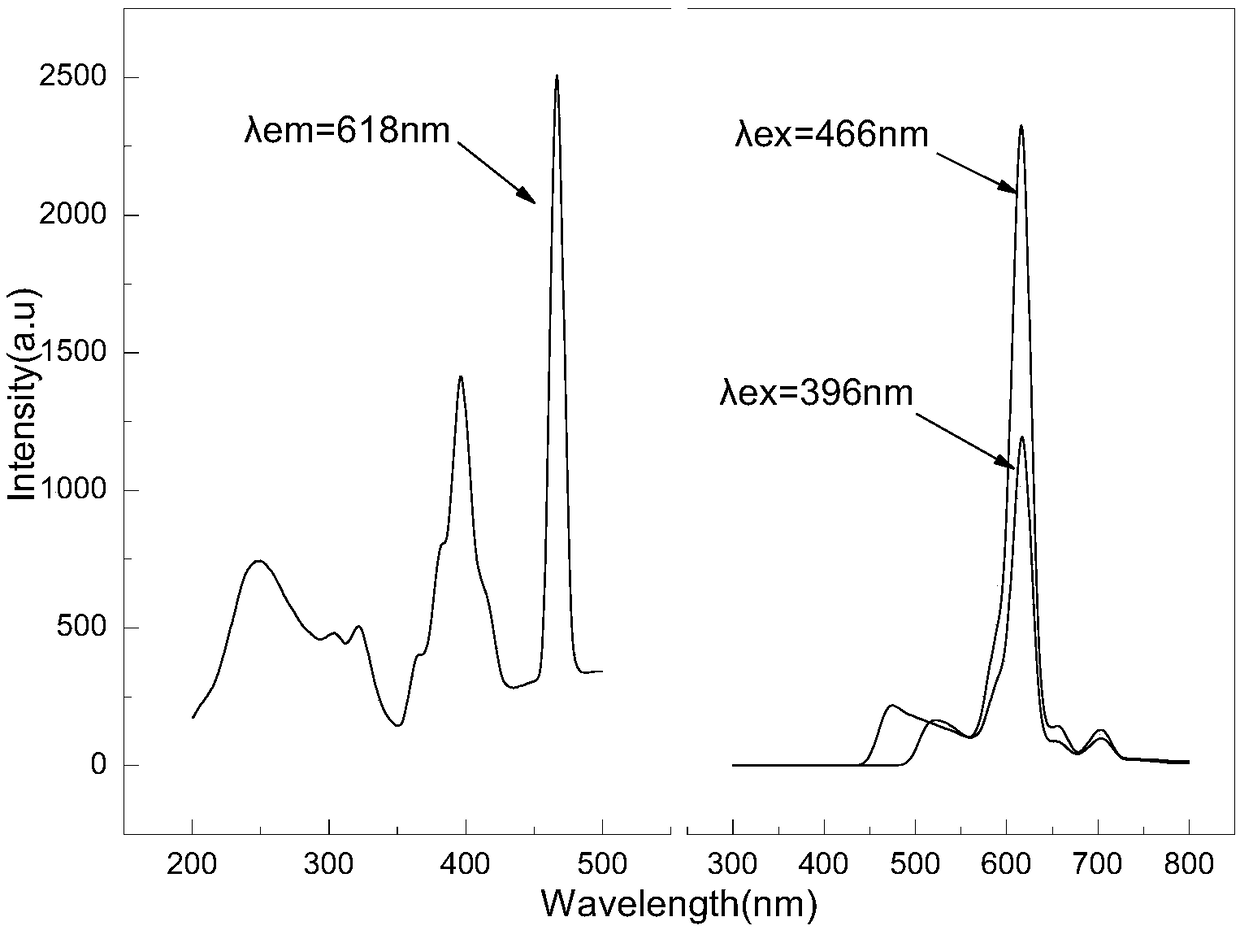
[0025] Weigh 2.224g of kaolin and K respectively with an analytical balance 2 CO 3 Powder 0.7864g, ensure that the mol ratio of potassium carbonate and kaolin is 1.2, potassium carbonate is made into a 6mol / L solution with deionized water; take a certain amount of europium nitrate powder, and make a 0.2mol / L solution with deionized water solution. Mix kaolin and potassium carbonate solution in a stirrer evenly, then add europium nitrate solution according to 5% of the theoretical mass of kalephine, mix the three evenly and dry at 100°C for 2 hours, and grind the dried mixture again to a uniform Put the powder into an alumina crucible, and sinter in an air atmosphere at 1000°C for 2 hours in a resistance furnace to obtain Eu 3+ Doped kacryptite-based fluorescent material.
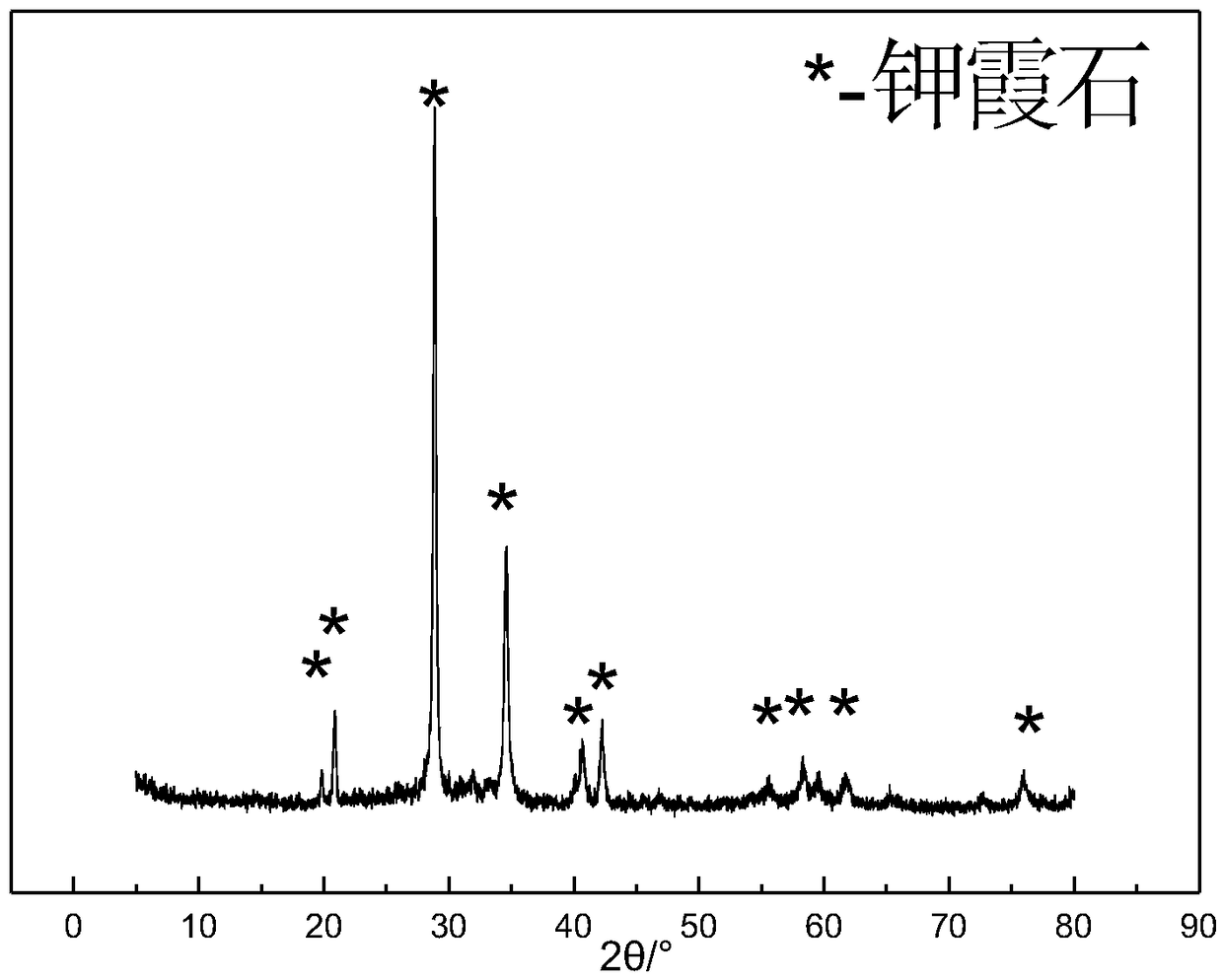
[0026] figure 1 It is the XRD pattern of the product synthesized in this example. It can be seen from the figure that the synthesized product is kalpheline KAlSiO4 phase, which is consistent with the sta...
Embodiment 2
[0030] Weigh 2.224g of kaolin and K respectively with an analytical balance 2 CO 3 Powder 0.7864g, ensure that the mol ratio of potassium carbonate and kaolin is 1.2, potassium carbonate is made into a 6mol / L solution with deionized water; take a certain amount of europium nitrate powder, and make a 0.2mol / L solution with deionized water solution. Mix kaolin and potassium carbonate solution evenly in a stirrer, then add europium nitrate solution according to 1% of the theoretical mass of kapineite to be formed, mix the three evenly and dry at 100°C for 20h, grind the dried mixture again to a uniform Put the powder into an alumina crucible, and in an air atmosphere, sinter in a resistance furnace at 700°C for 5 hours to obtain Eu 3+ Doped kacryptite-based red fluorescent material.
Embodiment 3
[0032] Weigh 2.224g of kaolin and K respectively with an analytical balance 2 CO 3 Powder 0.7864g, ensure that the mol ratio of potassium carbonate and kaolin is 1.2, potassium carbonate is made into a 6mol / L solution with deionized water; take a certain amount of europium nitrate powder, and make a 0.2mol / L solution with deionized water solution. Mix kaolin and potassium carbonate solution evenly in a stirrer, then add europium nitrate solution according to 1% of the theoretical mass of kalephine, mix the three evenly and dry at 100°C for 5 hours, and grind the dried mixture again until it becomes uniform Put the powder into an alumina crucible, and in an air atmosphere, sinter in a resistance furnace at 1100°C for 2 hours to obtain Eu 3+ Doped kacryptite-based red fluorescent material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com