Methods of treating depression using orexin-2 receptor antagonists
A depression, subject technology, used in pill delivery, pharmaceutical formulations, liquid delivery, etc., to solve problems such as unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
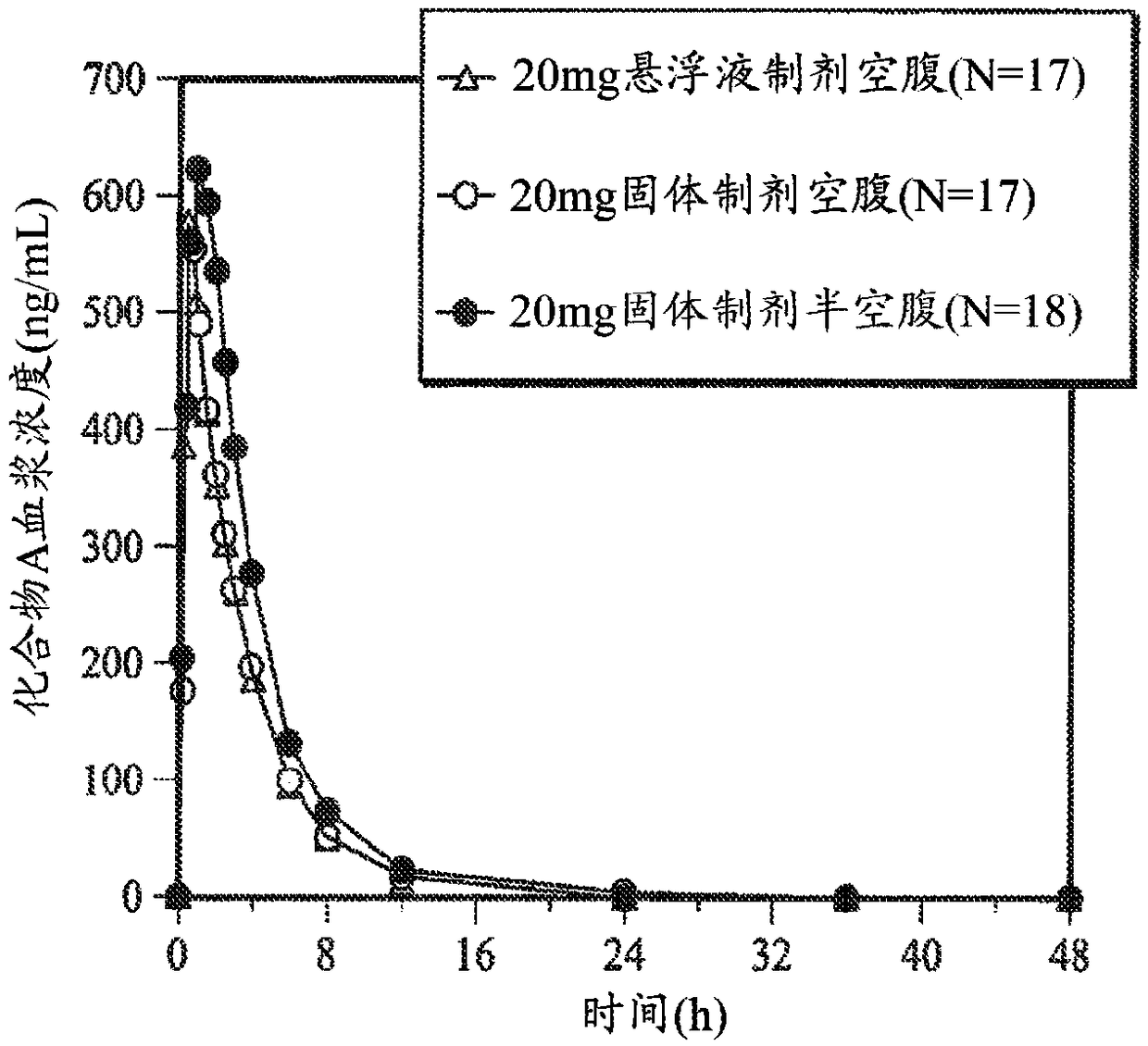
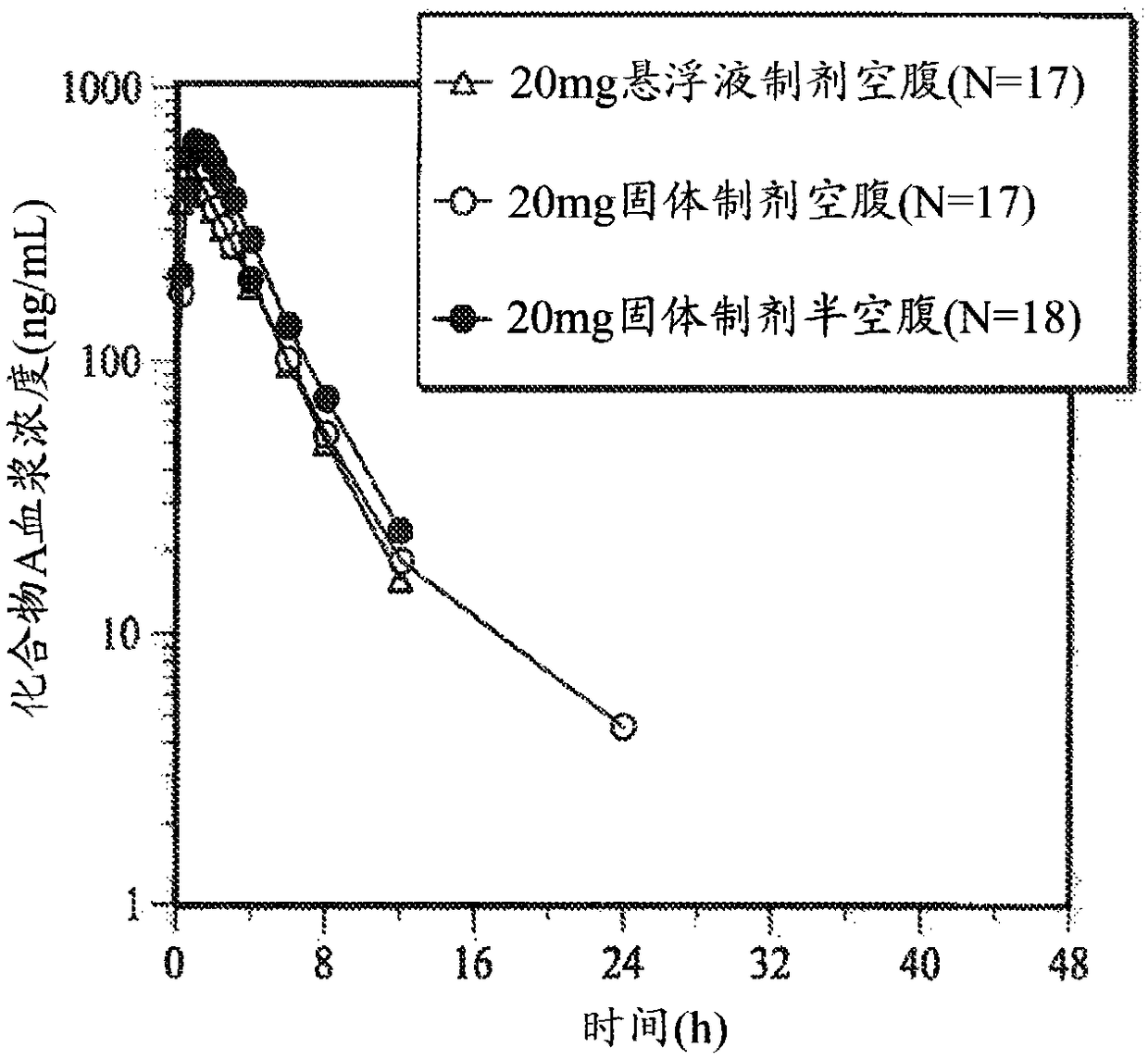
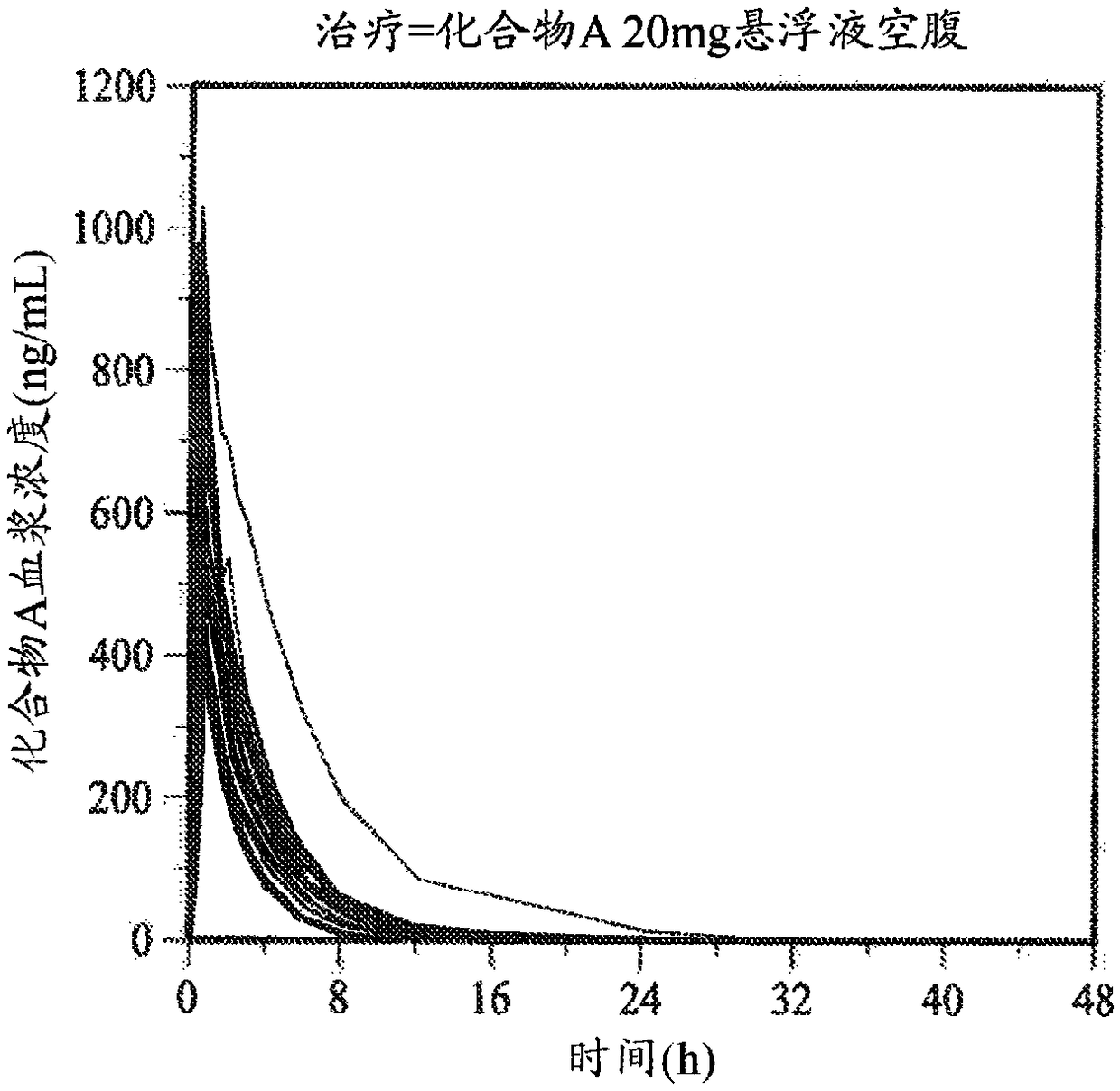
[0150] This example was performed to determine that [5(4,6-dimethyl-pyrimidin-2-yl)-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl]-(2-fluoro-2[ 1,2,3] Triazol-2-yl-phenyl)-methanone (compound A) plasma pharmacokinetics (PK) and biological utilization rate. The effect of semi-fasting conditions on the bioavailability of solid dosage formulations and the degree of tolerability of solid and oral suspension formulations is also discussed.
[0151] (i) Reagents and test parameters
[0152] [5(4,6-Dimethyl-pyrimidin-2-yl)-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl]-(2-fluoro-2-[1,2,3 ]triazol-2-yl-phenyl)-methanone (Compound A) was prepared as described in Method B in Example 107 of U.S. Pat. crystallization.
[0153] The internal standard is isotopically labeled [5(4,6-dimethyl-pyrimidin-2-yl)-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl]-(2-fluoro-2- [1,2,3]Triazol-2-yl-phenyl)-methanone, which has the following structure.
[0154]
[0155] The internal standard was prepared as described in Meth...
Embodiment 2
[0239] This example was conducted as a multicentre double-blind diphenhydramine and placebo controlled study. Men and women diagnosed with MDD between the ages of 18 and 64 (inclusive) were enrolled. At screening, subjects are on IDS-C 30 A total score of ≥30 corresponds to moderate to severe depression.
[0240] Blood and saliva are collected for evaluation of biomarkers, among other things. Venous blood samples (3 mL each) were collected under fasting conditions between 8:00 and 10:00 am for the determination of [5(4,6-dimethyl-pyrimidin-2-yl)-hexa Hydrogen-pyrrolo[3,4-c]pyrrol-2-yl]-(2-fluoro-2-[1,2,3]triazol-2-yl-phenyl)-methanone plasma concentration and correlation with immune Systemic activity-related biomarkers measuring hypothalamic-pituitary-adrenal (HPA) axis activation, neurotrophic factors, and metabolic factors. Pharmacokinetic (PK) blood samples were also collected. Plasma samples were analyzed to determine the concentration of Compound A using LC-MS / MS. S...
Embodiment 3
[0271] This example was performed to show that Compound A can be used in adjuvant therapy. Specifically, Compound A was administered to subjects diagnosed with MDD (i) as monotherapy and (ii) in combination with known antidepressants, and the subjects were assessed for depression using the HDRS17 and HAM-D6 scales symptom.
[0272] In Group 1, thirty-seven subjects were randomized (in a 2:1:1 ratio) to 20 mg Compound A, 25 mg diphenhydramine, or placebo q.d. in the evening after 10 days. In Group 2, ten subjects were randomized (in a 2:1:1 ratio) to 20 mg of Compound A, 25 mg of diphenhydramine, or placebo q.d. in the evening after 10 days. Each subject in Group 2 also took an amount of an antidepressant selected from duloxetine, citalopram, paroxetine, or sertraline, and as prescribed by their attending physician. Specified. To assess depressive symptoms in both groups, at screening and on day 11, i.e., the day after the 17 and HAM-D 6 Evaluations were performed independ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com