Preparation method for docetaxel impurity
A docetaxel and impurity technology, applied in the field of pharmaceutical preparation, can solve problems such as undetected impurities, and achieve the effects of reducing reaction steps, high reaction yield and few steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
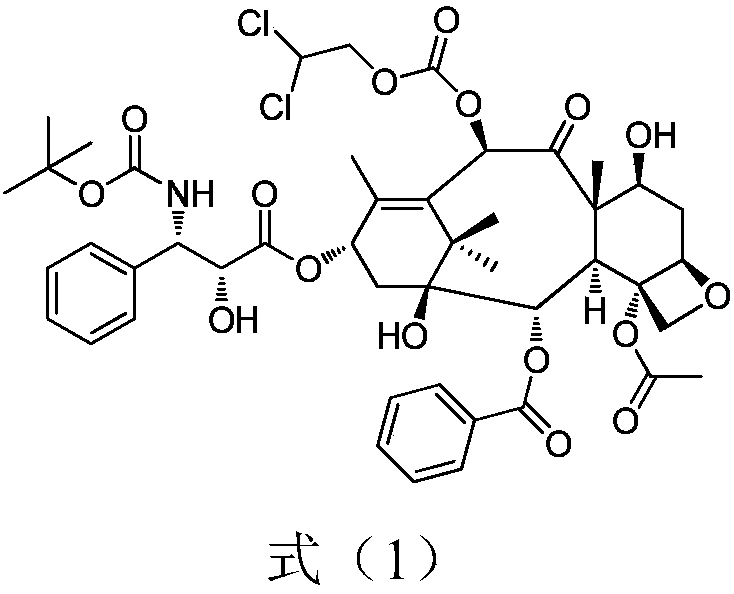
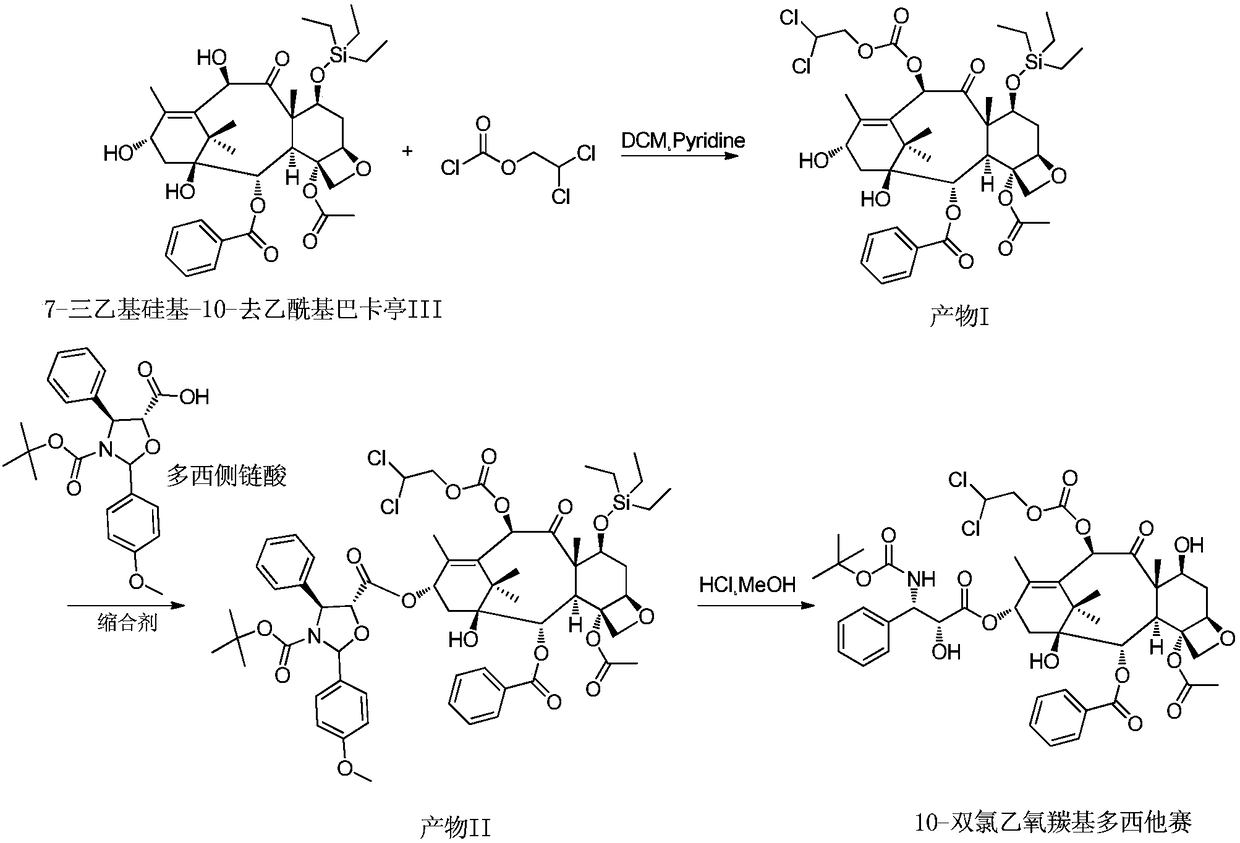
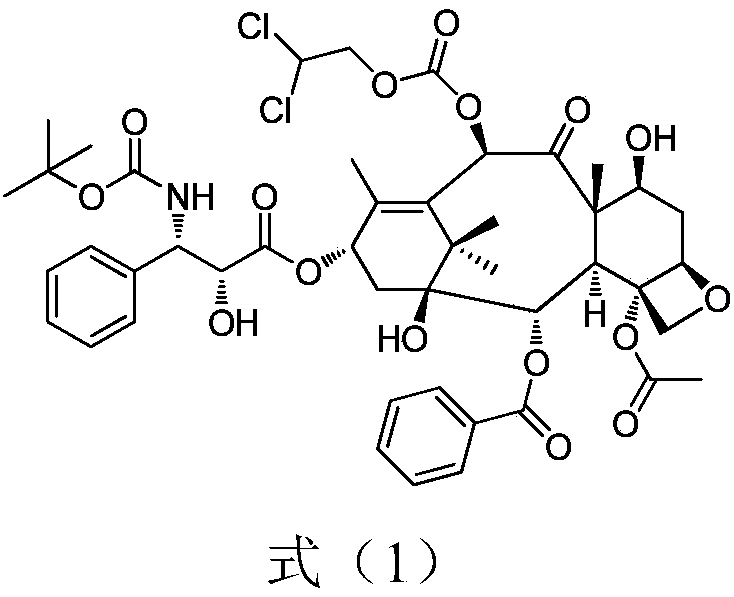
[0025] (1) Dissolve 7-triethylsilyl-10-deacetylbaccatin III (6.5g) in 32.5ml of dichloromethane and 65ml of pyridine, stir in a low-temperature bath, and dropwise add chloroformic acid-2 , 2-dichloroethyl ester (7.8g), continue to react at 0°C for 60min after dropping. Add 10ml of water to the reaction solution to quench the reaction, add hydrochloric acid to neutralize until the water phase becomes acidic, collect the organic phase and concentrate it under reduced pressure at 45°C until it is evaporated without solvent, add 30ml of toluene, continue to concentrate until a large amount of solids are precipitated, filter with suction, and dry Product I (7.5 g) was obtained with a yield of 95.0%.
[0026] (2) Product I (7.5g) was dissolved in 75ml toluene, and 4-dimethylaminopyridine (0.7g), dorsic acid (4.5g) and N,N-dicyclohexylcarboimide ( 3.3g, 1.7 equivalents), reacted at 15°C for 2h. The reaction solution was suction filtered, and the filtrate was washed with dilute hydr...
Embodiment 2
[0029] (1) Dissolve 7-triethylsilyl-10-deacetylbaccatin III (6.5g) in 32.5ml of dichloromethane and 13ml of pyridine, stir in a low-temperature bath, and dropwise add chloroformic acid-2 , 2-dichloroethyl ester (15.6g), continue to react at 0°C for 15min after dropping. Add 10ml of water to the reaction solution to quench the reaction, add hydrochloric acid to neutralize until the water phase becomes acidic, collect the organic phase and concentrate it under reduced pressure at 45°C until it is evaporated without solvent, add 30ml of toluene, continue to concentrate until a large amount of solids are precipitated, filter with suction, and dry Product I (7.8 g) was obtained with a yield of 98.9%.
[0030] (2) Dissolve the product I (7.8g) in 78ml of dichloromethane, add 4-dimethylaminopyridine (0.7g), dorsic acid (3.9g) and N,N-diisopropylcarbodi Imine (1.8g, 1.5eq) was reacted at 25°C for 1.5h. The reaction solution was suction filtered, and the filtrate was washed with dilu...
Embodiment 3
[0033] (1) Dissolve 7-triethylsilyl-10-deacetylbaccatin III (6.5g) in 32.5ml of dichloromethane and 30ml of pyridine, stir in a low-temperature bath, and dropwise add chloroformic acid-2 , 2-dichloroethyl ester (12.5g), continue to react at 0°C for 15min after dropping. Add 10ml of water to the reaction solution to quench the reaction, add hydrochloric acid to neutralize until the water phase becomes acidic, collect the organic phase and concentrate it under reduced pressure at 45°C until it is evaporated without solvent, add 30ml of toluene, continue to concentrate until a large amount of solids are precipitated, filter with suction, and dry Product I (7.3 g) was obtained with a yield of 92.5%.
[0034] (2) Dissolve the product I (7.3g) in 73ml toluene, add 4-dimethylaminopyridine (0.7g), dorsic acid (14.6g) and 1-ethyl-(3-dimethylamino Propyl)carbodiimide hydrochloride (3.5g, 2eq), react at 20°C for 1h. The reaction solution was suction filtered, and the filtrate was washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com