A seven-membered fused ring-like benzotriazole acceptor and its preparation method and application
A technology of benzotriazole and dinitrobenzotriazole, which is applied in the field of organic solar cell material preparation, can solve the problems of narrow optical bandgap and low photoelectric conversion efficiency, and achieve narrow optical bandgap and good production efficiency. Effect of film properties and high photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The synthetic route is expressed as follows:
[0078]
[0079]
[0080] The preparation method of BIC, its main steps are:
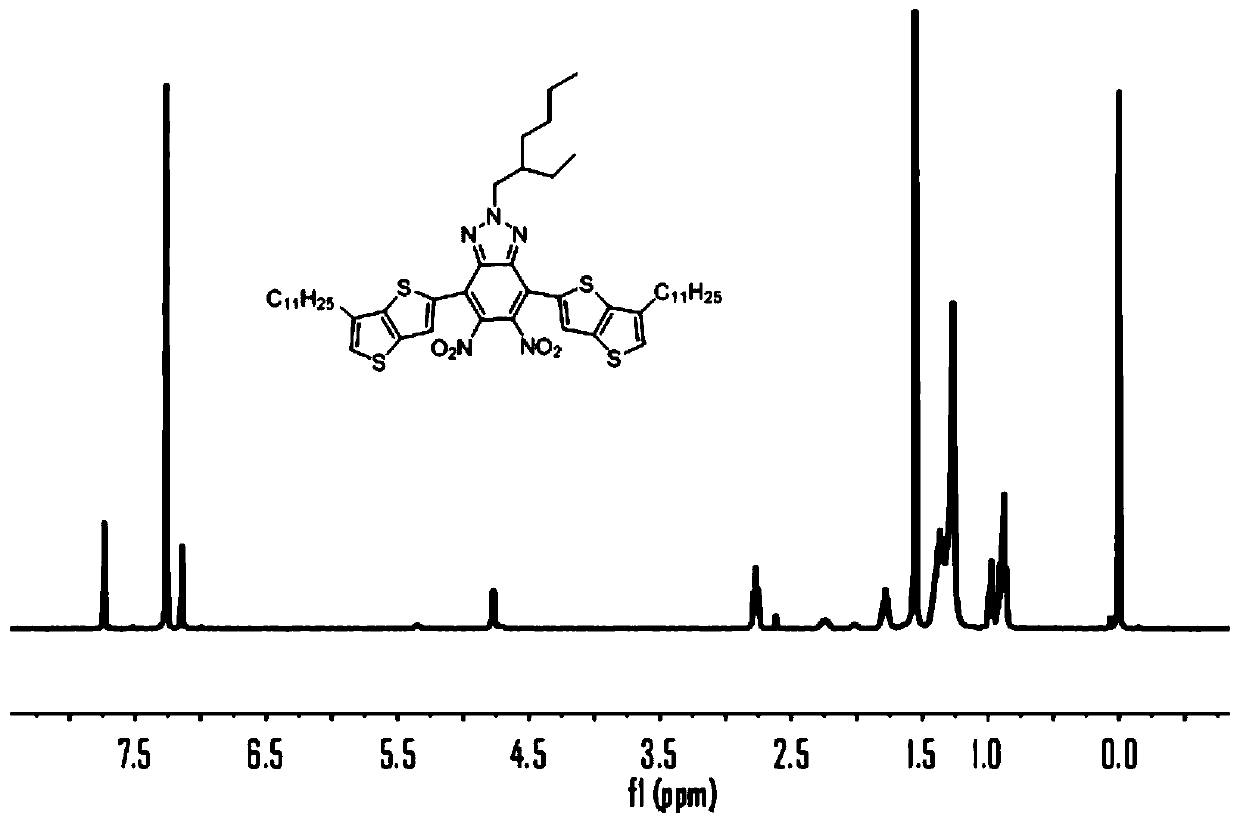
[0081] 1) 4,7-dibromo-2-(2-ethylhexyl)-5,6-dinitro-2H-benzo[d][1,2,3]triazole (2.5g, 5.22mmol ), tributyl(6-undecylthienothiophene)tin (6.46g, 17.3mmol) was dissolved in a flask filled with 30mL of anhydrous THF, under the protection of Ar, the gas was extracted three times, and the Pd(PPh 3 ) 2 Cl 2 (0.22g, 4mmol) was quickly added to the reaction system, and stirred under reflux at 70°C for 48h. After the reactant was cooled to room temperature, it was extracted with DCM, and the crude product was purified by a silica gel column, and the eluent was spin-dried to obtain a yellow solid (1). 1H NMR (400MHz, CDCl3) δ7.39(d, J=5.1Hz, 2H), 6.9(d, J=5.2Hz, 2H), 3.77(d, J=7.2Hz, 2H), 2.68(d, J =7.6Hz, 4H), 1.30(m, 1H), 1.50–0.90(m, 56H).
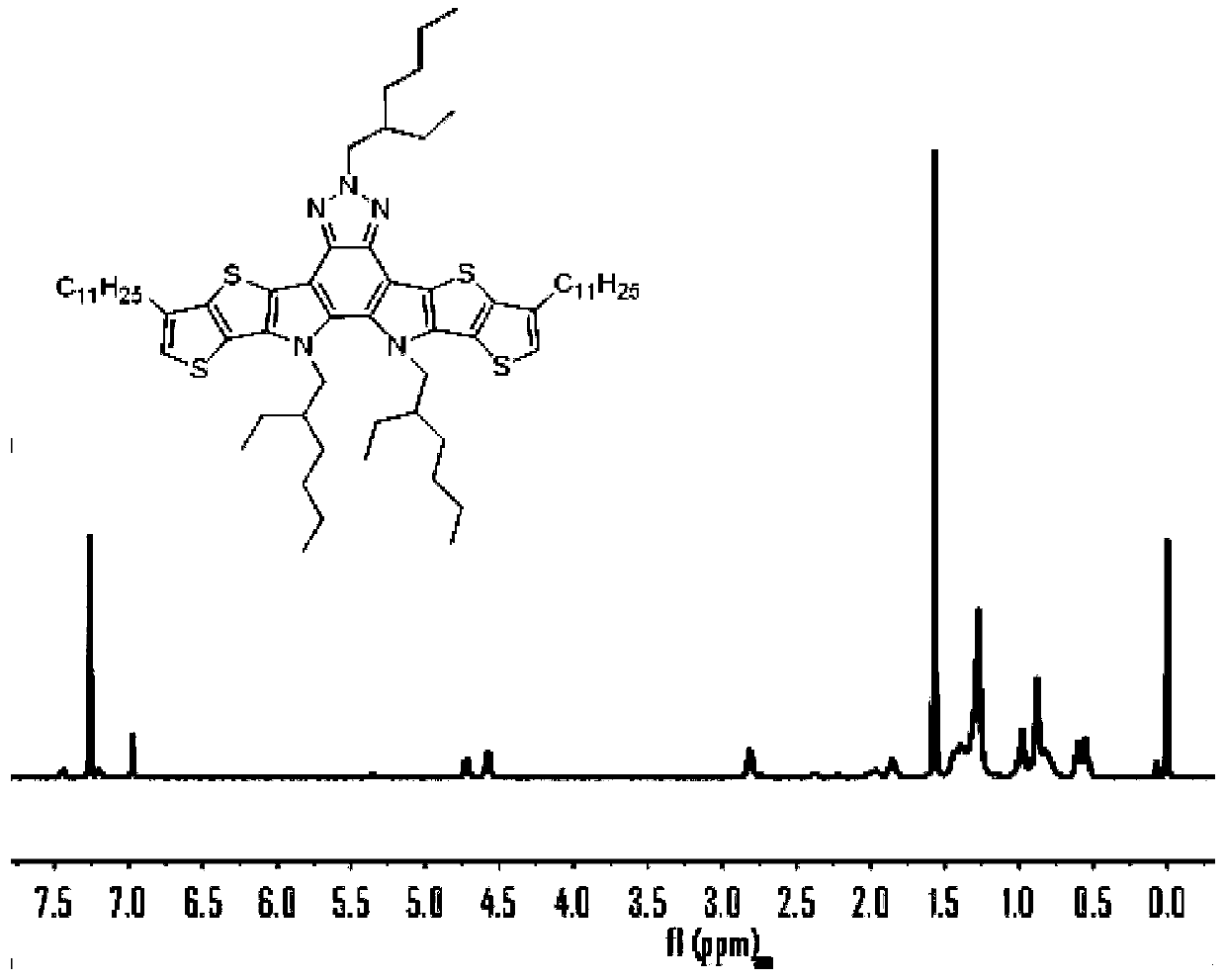
[0082] 2) Compound 1 (6.0g, 10mmol) was dissolved in a three-necked flask filled with o-dichlorobenzene...
Embodiment 2
[0095] Its main steps are with embodiment 1;
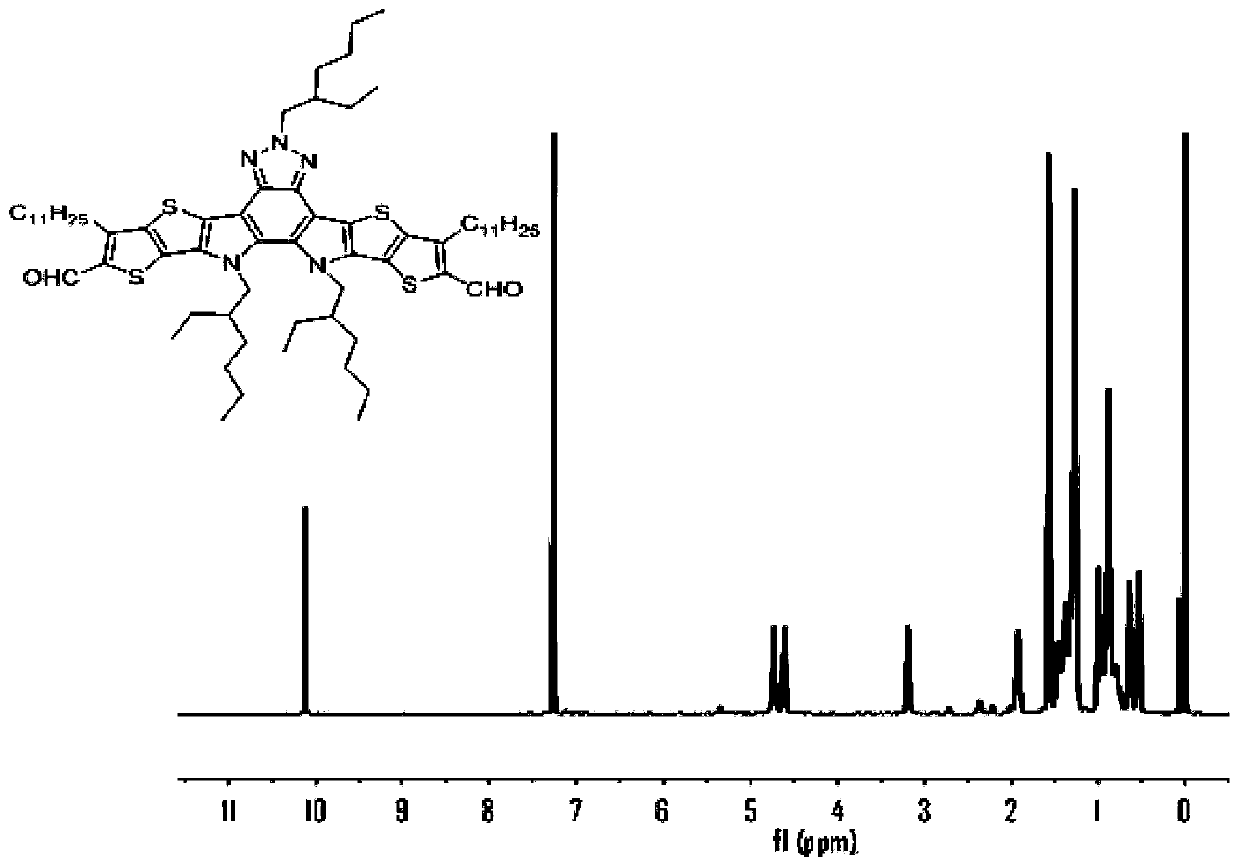
[0096] 1) Dissolve compound 3 (0.145g, 0.13mmol), 5,6-difluoro-3-(dicyanomethylene) indoketone (2FINCN) (0.27g, 1.3mmol), pyridine (1mL in a In a one-necked bottle of 30 mL of chloroform, under the protection of Ar, stirred overnight at 65 °C under reflux, cooled the reaction to room temperature, extracted with DCM, and purified the crude product with a silica gel column to obtain a dark blue solid (BFIC). (0.08 g, 40%). 1 H NMR (400MHz, CDCl 3 )δ9.34(s,1H),8.54(dd,J=5.7,2.9Hz,1H),7.76(dt,J=7.3,3.7Hz,1H),7.84–7.71(m,4H),3.90(d , J = 7.2Hz, 4H), 3.77 (d, J = 7.2Hz, 2H), 2.68 (d, J = 7.6Hz, 4H), 1.30 (m, 3H), 1.50–0.90 (m, 80H).
[0097]
[0098]
[0099] Device Fabrication and Photovoltaic Performance
[0100] Device preparation:
[0101] The donor and acceptor materials were weighed according to different ratios, and chromatographically pure chloroform was selected as the solvent. Add different additives according to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com