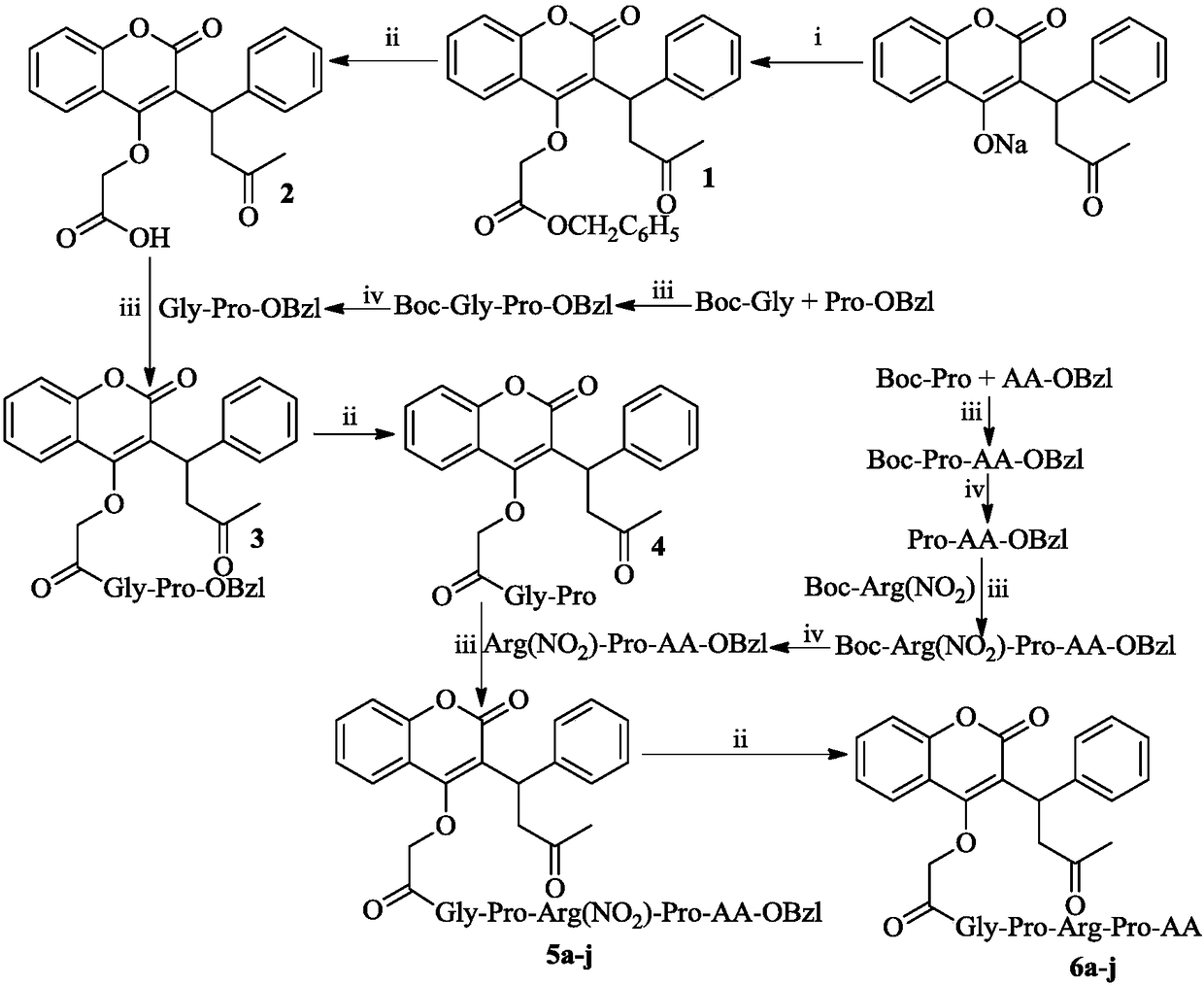
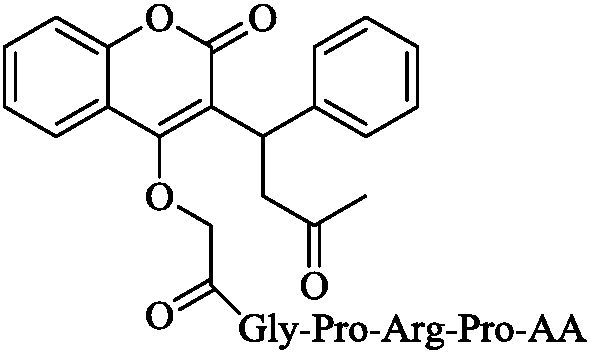
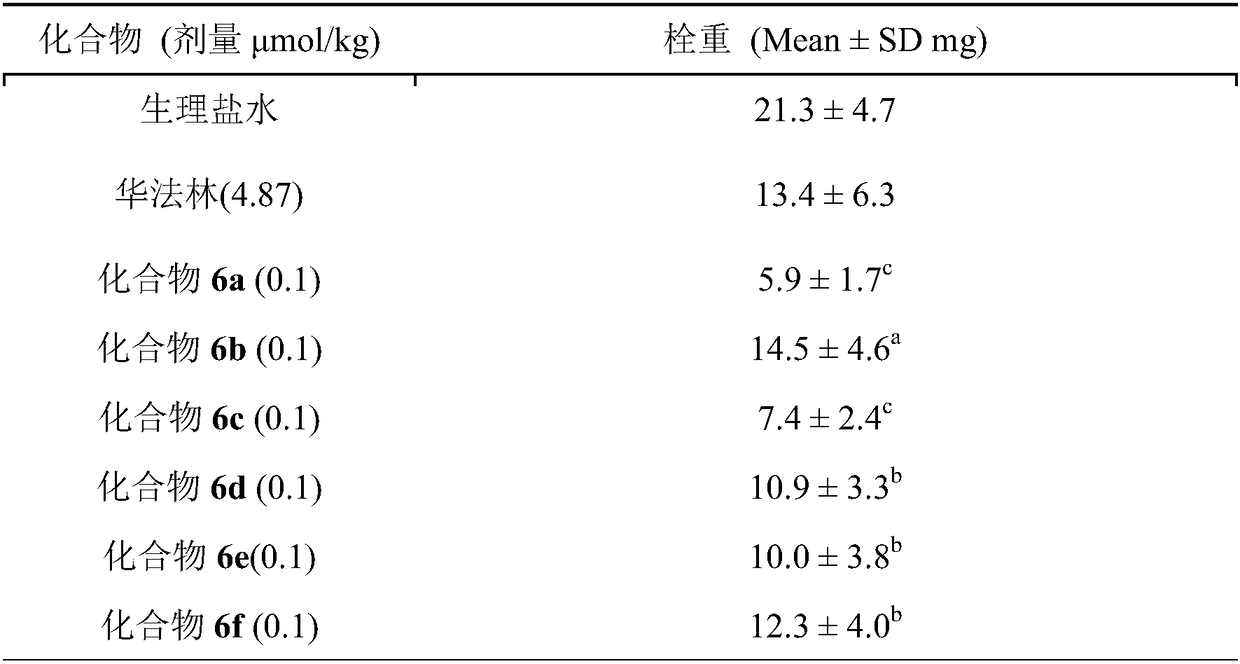
Gly-Pro-Arg-Pro-containing pentapeptide-modified warfarin, synthesis, activity and applications thereof
A technology of warfarin and liquid phase method, applied in the preparation of anti-venous thrombosis drugs, warfarin-4-O-acetyl-Gly-Pro-Arg-Pro-AA, anti-venous thrombosis active field, can Solve the problems of not being able to obtain anti-venous thrombosis activity, fatal bleeding, and narrow warfarin safety window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 prepares warfarin-4-O-acetic acid benzyl ester
[0017] 26.48g (80.00mmol) of warfarin and 400mL of acetone were stirred at 45°C until the warfarin was dissolved. 14 mL (88 mmol) benzyl bromoacetate was added to the obtained solution, and stirring was continued at 45° C. for 96 h. Thin layer chromatography (TLC, petroleum ether / ethyl acetate=2 / 1) showed that the reaction was complete. The reaction solution was filtered off, and the filtrate was concentrated under reduced pressure. The obtained pale yellow oil was purified by silica gel column chromatography (petroleum ether / ethyl acetate=8 / 1) to obtain 19.77 g (54%) of the title compound as a colorless solid. ESI-MS(m / e):457[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 )δ / ppm=7.89(dd,J 1 =3.0Hz,J 2 =9.0Hz,1H),7.63(dt,J 1 =3.0Hz,J 2 =9.0Hz, 1H), 7.43~7.31(m, 9H), 7.24(t, J=9.0Hz, 2H), 7.15(tt, J=9.0Hz, 1H), 5.26(s, 2H), 5.61(s ,1H),5.02(d,J=15.0Hz,1H),4.85(d,J=15.0Hz,1H),4.97(t,J=9.0Hz,1H),3.45(dq,J 1 =9.0Hz,...
Embodiment 2
[0018] Embodiment 2 prepares warfarin-4-O-acetic acid
[0019] 19.77 g (43.36 mmol) of benzyl warfarin-4-O-acetate was dissolved in 150 mL of tetrahydrofuran, and then suspended with 4.94 g of palladium on carbon (Pd / C). Pass hydrogen gas inside for 72h. TLC (petroleum ether / ethyl acetate=2 / 1) showed that the reaction was complete. The Pd / C was filtered off and the filtrate was concentrated under reduced pressure to afford 15.58 g (98%) of the title compound as a colorless solid. ESI-MS(m / e):367[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 ):δ / ppm=12.86(s,1H),7.90(d,J=6.0Hz,1H),7.63(t,J=6.0Hz,1H),7.43~7.34(m,4H),7.27(t, J=9.0Hz, 2H), 7.17(t, J=9.0Hz, 1H), 4.99(t, J=9.0Hz, 1H), 4.75(dd, J 1 =15.0Hz,J 2 =30.0Hz, 2H), 3.54~3.47(m, 2H), 2.14(s, 3H).
Embodiment 3
[0020] Embodiment 3 prepares Boc-Gly-Pro-OBzl
[0021] Dissolve 7.730g (44.15mmol) Boc-Gly in 100mL of anhydrous tetrahydrofuran, add 5.940g (44.00mmol) 1-hydroxybenzotriazole (HOBt) and 9.888g (48.00mmol) dicyclohexyl to the solution at 0°C Carbodiimide (DCC), stirred at 0°C for 30min and added 9.665g (40.00mmol) HCl·Pro-OBzl to it. The pH of the reaction solution was adjusted to 9 with N-methylmorpholine, stirred at room temperature for 17 h, and TLC (dichloromethane / methanol=20 / 1) showed that the reaction was complete. The insoluble matter in the reaction solution was filtered off, the filtrate was concentrated under reduced pressure, the residue was dissolved in 150 mL ethyl acetate, the colorless solid was filtered off, and the filtrate was washed with saturated NaHCO 3 Solution washing (50mL×3), saturated NaCl solution washing (50mL×3), 5% KHSO 4 Solution washing (50mL×3), saturated NaCl solution washing (50mL×3), saturated NaHCO 3 Solution washing (50mL×3), saturated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com