Norbornene derivative based homopolymer containing heterogeneous sugar units and synthetic method of homopolymer
A technology for norbornene and a synthesis method, which is applied in the field of sugar-containing polymer synthesis, can solve problems such as complex multi-step operations of block copolymerization, and achieve the effects of broadening synthesis routes, regular structure and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
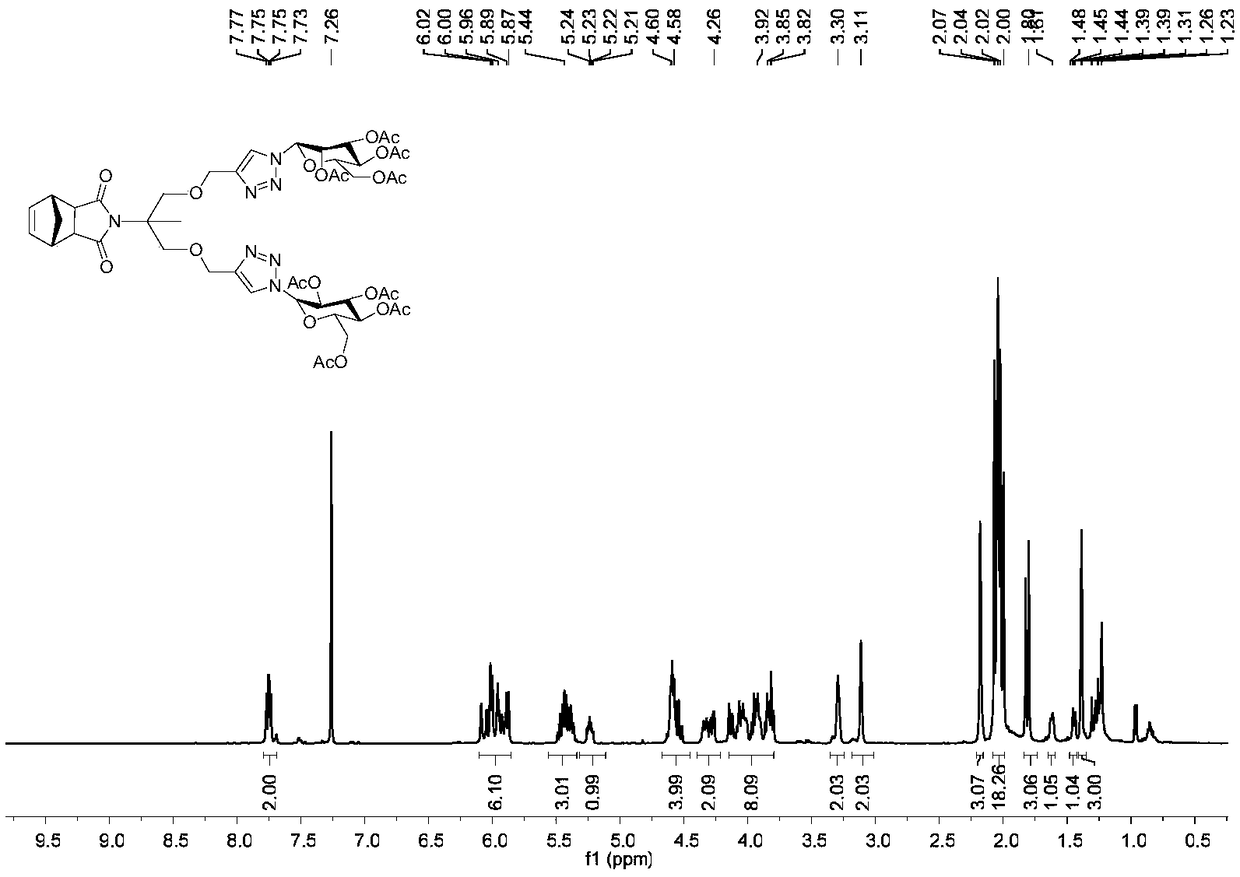
Examples
Embodiment 1
[0044] 1. Synthesis of norbornene dicarboxamide propylene glycol NB-2OH (1)
[0045] Take the three-neck round bottom bottle cooled after high temperature treatment, add norbornene dianhydride (5g, 30mmol) and aminomethylpropanediol (3.15g, 30mmol), and add 150mL of toluene. Connect the h-type water separator at the mouth of the bottle, connect the serpentine condenser above it, and reflux the reaction in an oil bath at 135°C for 16 hours. After the reaction, the toluene is distilled off under reduced pressure. Purify directly through a silica gel column to obtain 2.8 g of a white solid with a yield of 37%. 1 H NMR (500MHz, CDCl3 ):δ=1.17(s,3H),1.49(d,J=8.7Hz 1H),1.57(d,J=8.8Hz,1H),3.23(m,2H),3.40(m,2H),3.58– 3.68(m,4H),4.19(d,J=11.8,2H),6.15(s,2H).
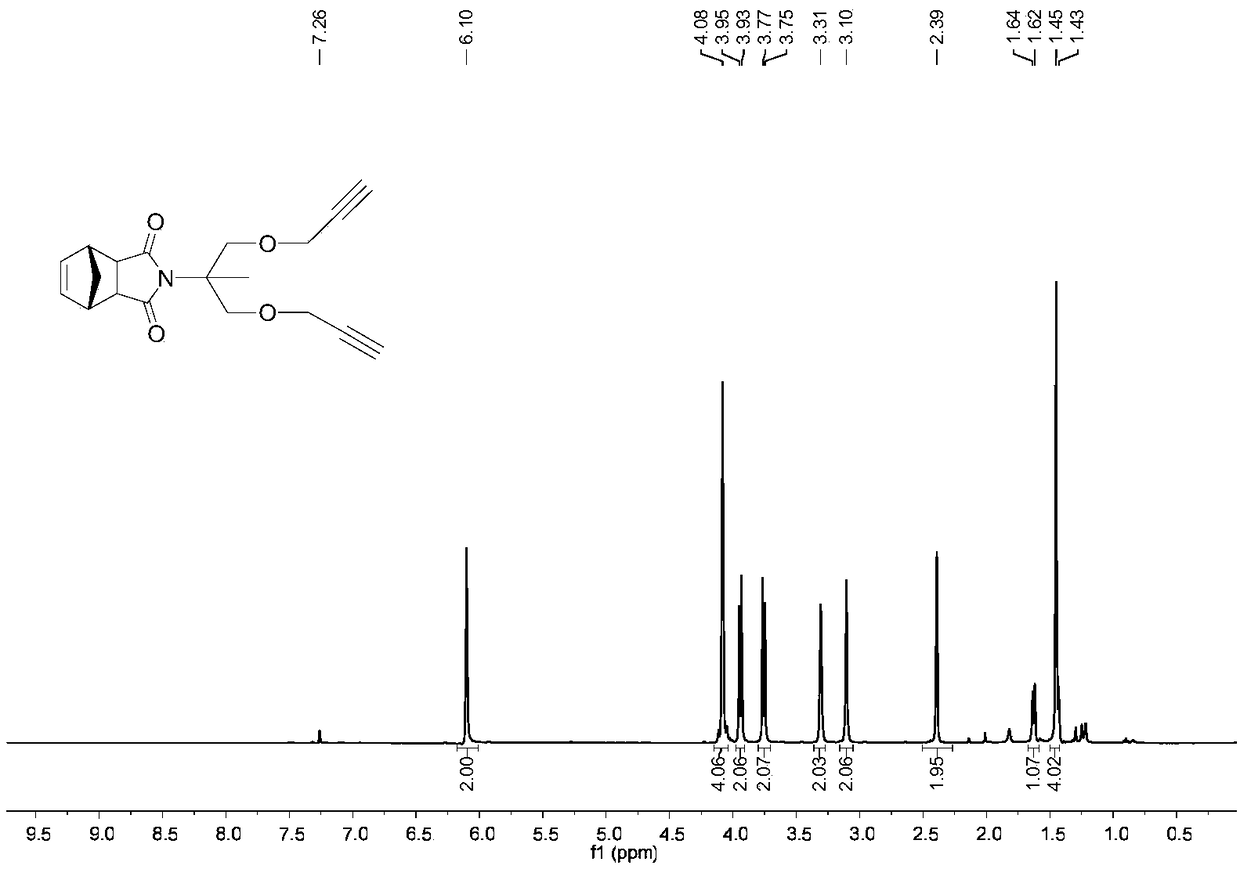
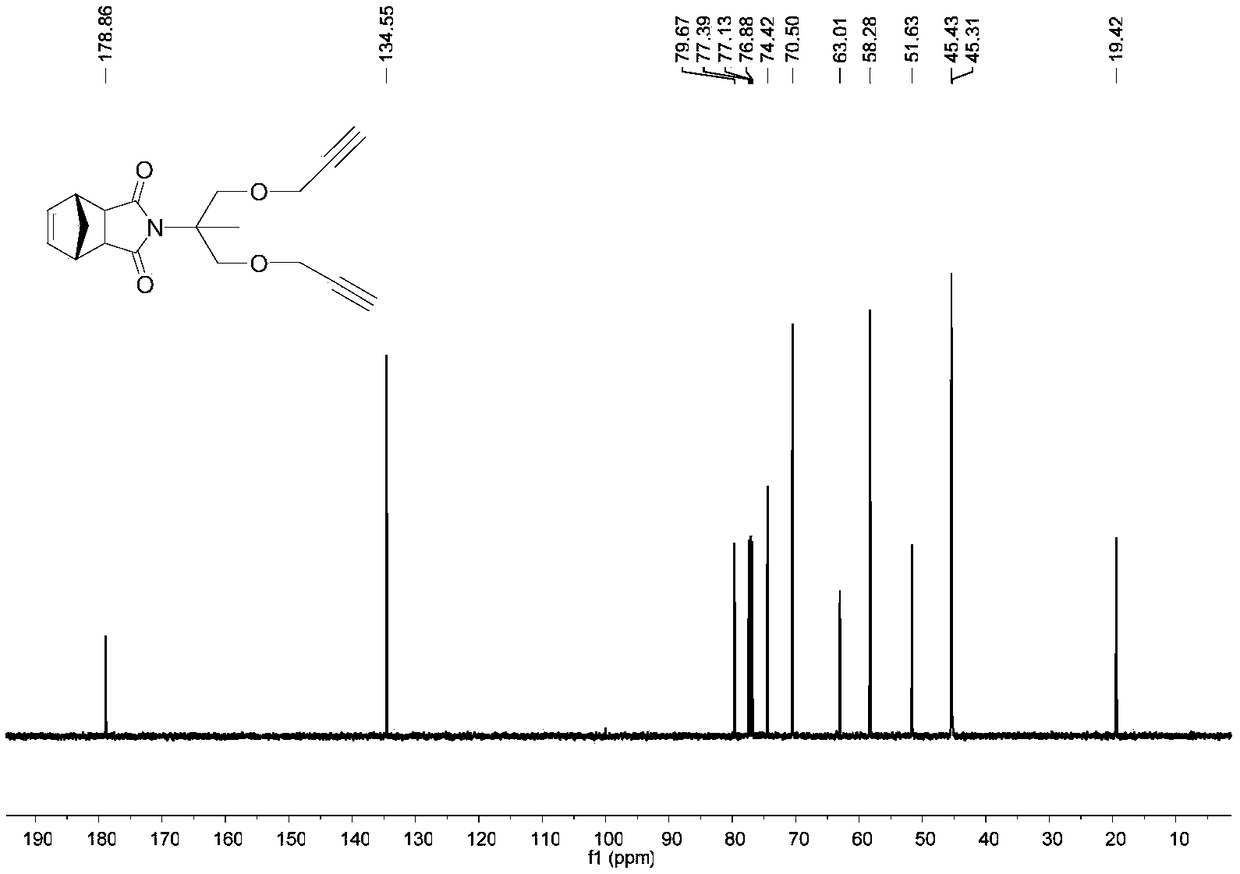
[0046] 2. Synthesis of 2,2-diyne-norbornene dicarboxamide ethylene glycol NB-2Alkyne (2)
[0047] Add norbornenedicarboxamide propylene glycol (1.88g, 7.5mmol) into a dry single-necked round-bottomed reaction flask, then add 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com