G-tetramer covalently coupled DNA molecule, DNA self-transfection kit and application
A DNA molecule and covalent coupling technology, applied in the field of DNA transfection, can solve the problems of complicated operation, concentration requirements and immunogenicity of the microinjection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
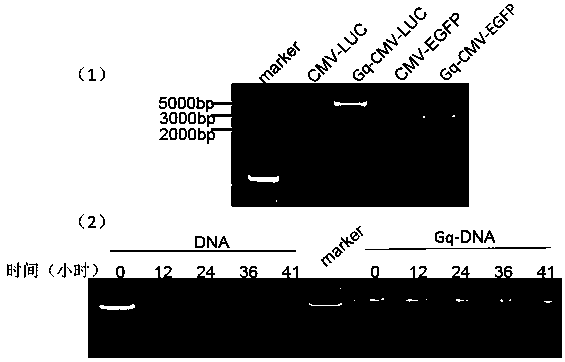
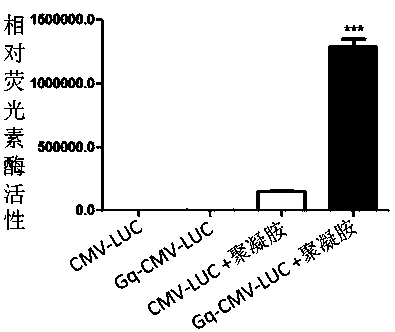
[0070] Example 1: Gq mediates luciferase gene self-infection
[0071] Taking the luciferase gene as an example, this DNA self-transfection kit is described.
[0072] step 1
[0073] The luciferase gene was constructed on the pCMV-tag2A vector.
[0074] step 2
[0075] First design a pair of primers (primer 1) (upstream of primer 1: 5-TTTTGCTCACATGTTCTTTC-3 downstream: 5-ATTTACGCGTTAAGATA-3) to amplify CMV-LUC-polyA, and precipitate and recover it as a template for the next PCR and experimental control .
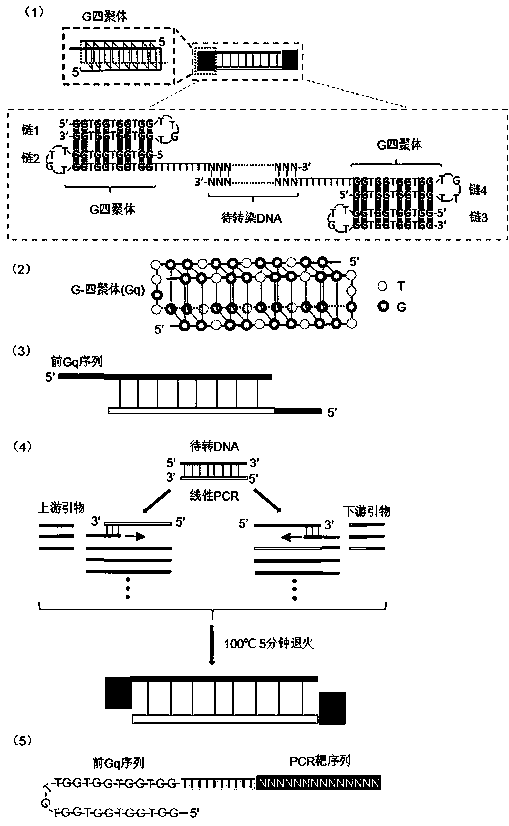
[0076] Then design a pair of primers (primer 2) (primer 2 upstream primer 5-GGTGGTGGTGGTTGTGGTGGTGGTGGttttttttTTTTGCTCACATGTTCTTTC-3 downstream primer 5-GGTGGTGGTGGTTGTGGTGGTGGTGGttttttttATTTACGCGTTAAGATA-3), its 5' end has the sequence of nucleolin affinity adapter AS1411 5'( GGTGGTGGTGGTTGTGGTGGTGGTGG)-3', the structure of primer 2 is as figure 1As shown in (5), the upstream and downstream primers were respectively named as primer 2-F and primer 2-R. Then use the prod...
Embodiment 2
[0089] Example 2: Gq mediates green fluorescent protein (EGFP) gene self-infection
[0090] Taking the GFP gene as an example, this DNA self-transfection kit is described.
[0091] step 1
[0092] First design a pair of primers (primer 1) (upstream of primer 1: 5-TTTTGCTCACATGTTCTTTC-3 downstream: 5-ATTTACGCGTTAAGATA-3) using the plasmid pEGFPC1 as a template to amplify CMV-GFP-polyA and carry out precipitation recovery as the next step Template for PCR and experimental controls.
[0093] Then design a pair of primers (primer 2) (primer 2 upstream primer 5-GGTGGTGGTGGTTGTGGTGGTGGTGGttttttttTTTTGCTCACATGTTCTTTC-3 downstream primer 5-GGTGGTGGTGGTTGTGGTGGTGGTGGttttttttATTTACGCGTTAAGATA-3), its 5' end has the sequence of nucleolin affinity adapter AS1411 5'( GGTGGTGGTGGTTGTGGTGGTGGTGG)-3', the structure of primer 2 is as figure 1 As shown in (5), the upstream and downstream primers were respectively named as primer 2-F and primer 2-R. Then use the above linear product CMV-GFP ...
Embodiment 3
[0102] Example 3: Gq-mediated autoinfection of random DNA fragments prestained with dye YOYO-1
[0103] Taking a piece of random DNA with a length of about 800bp as an example, the efficiency and universality of this DNA autotransfection kit are illustrated.
[0104] step 1
[0105] First design a pair of primers (primer 1) (upstream of primer 1: 5-CATCGCATTGTCTGAGTAGGTG-3 downstream: 5-CGAGAAAGGAAGGGAAGAAAG-3) to amplify this random fragment using the plasmid px601 as a template, and carry out precipitation recovery as a template for the next PCR and experimental controls.
[0106] Then design a pair of primers (primer 2) (primer 2 upstream primer 5-GGTGGTGGTGGTTGTGGTGGTGGTGGTTTTTTTTTTGATTGGGAAGAGAATAGCAGGCAT-3 downstream primer 5-GGTGGTGGTGGTTGTGGTGGTGGTGGTTTTTTTTGCTACAGGGCGCGTACTATGGTT-3), its 5' end has nucleolin affinity Gq precursor sequence 5'-( GGTGGTGGTGGTTGTGGTGGTGGTGG)-3', the structure of primer 2 is as figure 1 (5) shown. Then use the above linear DNA as a tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com