A method for preparing 2,2-dichlorophenylacetonitrile
A technology of dichlorophenylacetonitrile and phenylacetonitrile, which is applied in the preparation of carboxylic acid nitriles, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of high equipment requirements, unfavorable control, large energy consumption, etc., and achieve good safety , low equipment requirements and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 1171g (10mol) phenylacetonitrile to the first reactor, heat to 110°C, and add another 1171g (10mol) benzylnitrile to the second reactor, also heat to 110°C, pass 1455g (20.5mol) of chlorine gas into the first reaction kettle, and continue heating under normal pressure at 110°C to react for 20 hours; the tail gas generated by the reaction is first passed into the second reaction kettle, and finally passed to the hydrochloric acid absorption tower. The liquid in the first reaction kettle was detected by GC, and the conversion rate of phenylacetonitrile was 99.4%. The liquid in the first reaction kettle was distilled under reduced pressure to obtain 1843 g of product 2,2-dichlorophenylacetonitrile, with a yield of 99.1%.
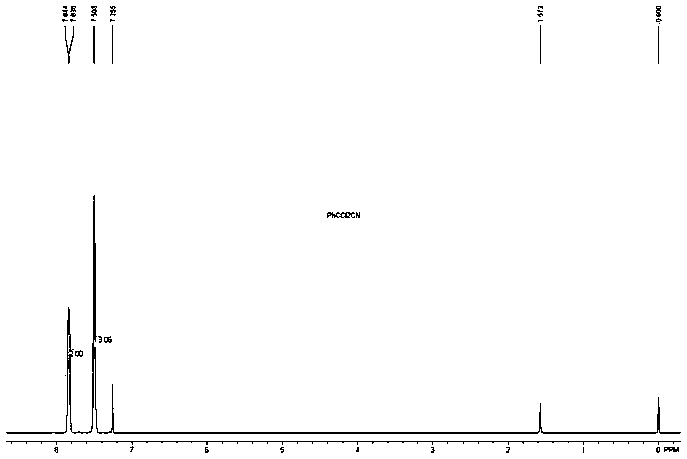
[0040] 2,2-Dichlorophenylacetonitrile NMR identification, the spectrum is as follows figure 1 Shown: 1 H NMR (400MHz, CDCl 3 ,δ,ppm):7.89-7.80(m,2H),7.55-7.45(m,3H).
Embodiment 2
[0042] Add 1171g (10mol) phenylacetonitrile to the first reaction kettle, heat to 100°C, and add another 1171g (10mol) benzylnitrile to the second reaction kettle, also heat to 100°C, pass chlorine gas 1475g (20.8mol) into the first reaction kettle, and continue to heat and react at normal pressure for 28 hours; the tail gas generated by the reaction is first passed into the second reaction kettle, and finally passed to the hydrochloric acid absorption tower. The liquid in the reaction kettle was detected by GC, and the conversion rate of phenylacetonitrile was 99.6%. The liquid in the first reaction kettle was distilled under reduced pressure to obtain 1850 g of the product 2,2-dichlorophenylacetonitrile, with a yield of 99.5%.
Embodiment 3
[0043] Embodiment 3: (cycle method)
[0044] The material in the second reactor in Example 2 (1171g (10mol) benzyl nitrile that has passed through last first reactor tail gas) is transferred in the first reactor, is heated to 100 ℃, simultaneously other 1171g (10mol) phenylacetonitrile is added to the second reaction kettle, also heated to 100°C, 1475g (20.8mol) of chlorine gas is passed into the first reaction kettle, and the heating reaction is continued at normal pressure for 28 hours; the tail gas generated by the reaction is first passed into the second reaction kettle In the kettle, it is finally passed to the hydrochloric acid absorption tower. The liquid in the reaction kettle was detected by GC, and the conversion rate of phenylacetonitrile was 99.8%. The liquid in the first reaction kettle was distilled under reduced pressure to obtain 1850 g of product 2,2-dichlorophenylacetonitrile, with a yield of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com