Nano-amplified self-reference probe for in-situ imaging of microRNA in living cells and preparation method thereof
A living cell and large self-reference technology, applied in the field of nano-amplified self-reference probes and its preparation, can solve problems such as difficult to maintain data stability and reliability, poor stability of nucleic acid probes, difficult miRNA signals, etc., to achieve Effects of fluorescence imaging and relative quantitative detection, improvement of detection specificity, and improvement of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
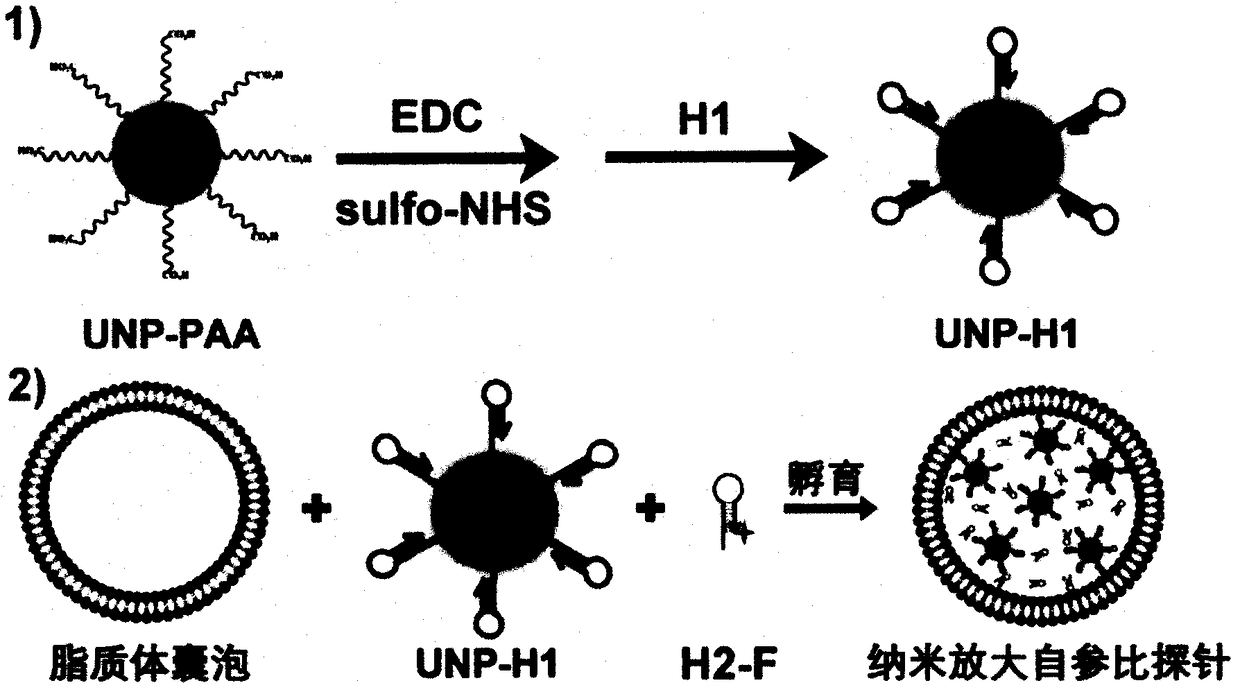
[0020] Example 1: Combining figure 1 , to prepare probes.
[0021] The probe preparation process is divided into two steps: 1) preparation of UNP-H1, and 2) preparation of probe by wrapping UNP-H1 and H2-F with lipo-3000.
[0022] 1) Preparation of UNP-H1: Taking the detection of miR21 in living cells as an example, the nucleotide sequence of H1 is 5’NH 2 - TCAACATCAGTCTGATAAGCTACCATGTGTAGATAGCTTTATCAGACT; H2 is TAAGCTATCTACACATGGTAGCTTATCAGACTCCATGTGTAGA-F-3'. UNP to NaYF 4 : Er / Gd / Yb@NaGdF 4 For example, EEB1 is at 530-560nm, and EEB2 is at 640-670nm. F Take AF555 as an example, its absorption peak overlaps with EEB1. 15.3 mg EDC (final concentration: 0.4 μM) and 4.34 mg sulfo-NHS (final concentration: 0.1 μM) were quickly added to 200 μL of PBS solution containing 1 mg / mL PAA-UNP, and shaken at room temperature for 4 h. Carboxyl-activated PAA-UNP was prepared by removing excess EDC and sulfo-NHS by centrifugation and redispersing to 200 μL. H1 was then added to the a...
Embodiment 2
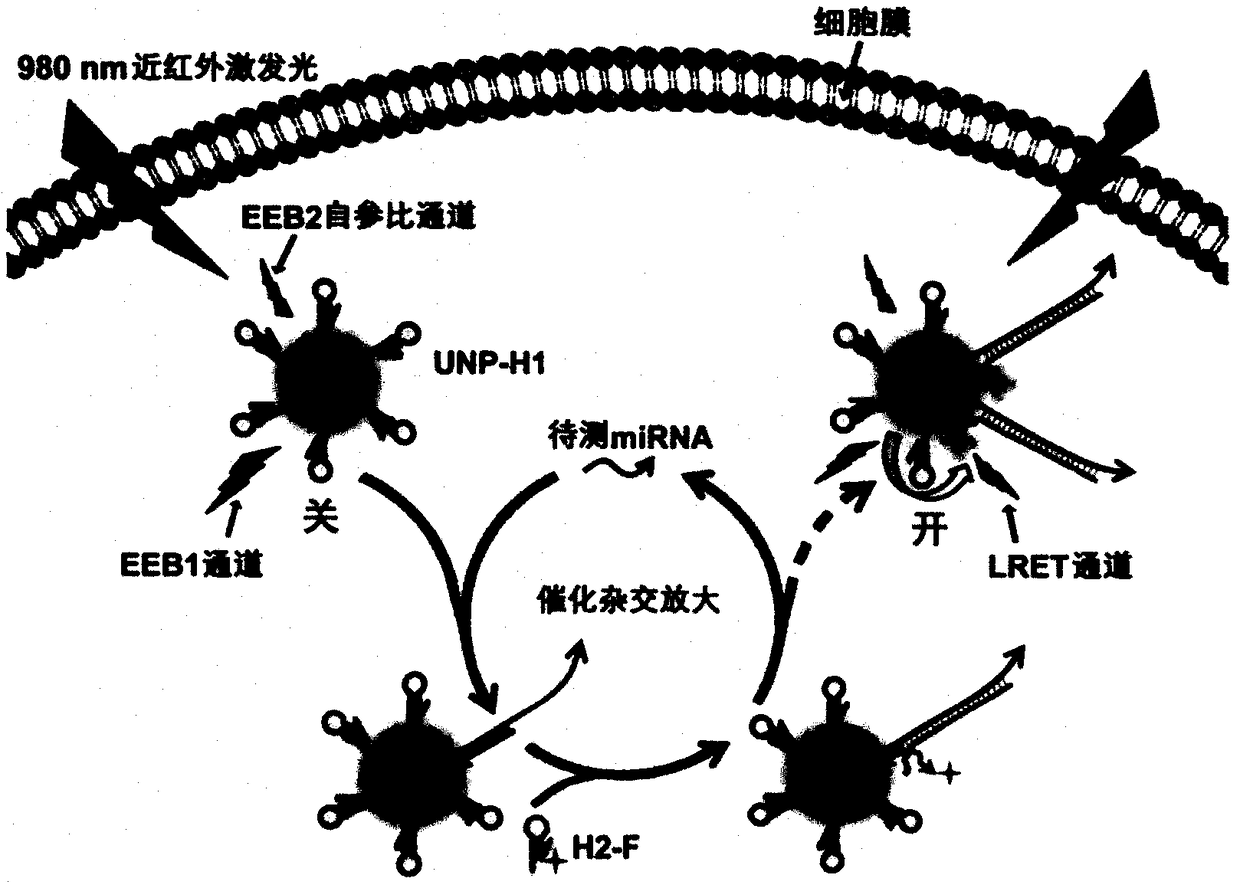
[0024] Example 2: Combining figure 2 , using the nano-amplified self-reference probe for in situ imaging and relative quantification of miRNAs in living cells.
[0025] Taking the detection of miR21 in MDA-MB-231 breast cancer cells as an example, the concentration of 0.5mL was 1×10 6 mL -1 The cell suspension was seeded in a 20-mm four-well confocal culture dish and cultured for 24 hours. Then, the nano-amplified self-reference probe prepared above was added to the culture dish (150 μL / well), and after co-incubating with the cells for 6 h, under the excitation of 980 nm near-infrared light, the LRET channel was imaged using a laser confocal fluorescence microscope. The fluorescence distribution map of the miRNA to be tested in cancer cells can be obtained. Read the signal intensity of LRET and EEB2 channels in single cell area respectively by confocal software, and calculate its ratio (I LRET / I EEB2 ), this ratio represents the expression level of the miRNA to be teste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com