Difunctional hydroxyl iron oxide desulphurization agent and preparation method thereof
A technology of iron oxyhydroxide and desulfurization agent, which is applied in the direction of chemical instruments and methods, separation methods, and dispersed particle separation, can solve the problems of high desulfurization cost, single desulfurization performance of desulfurization agent, and complex production process, so as to save investment and widely Industrial application prospects, the effect of simplifying the desulfurization process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
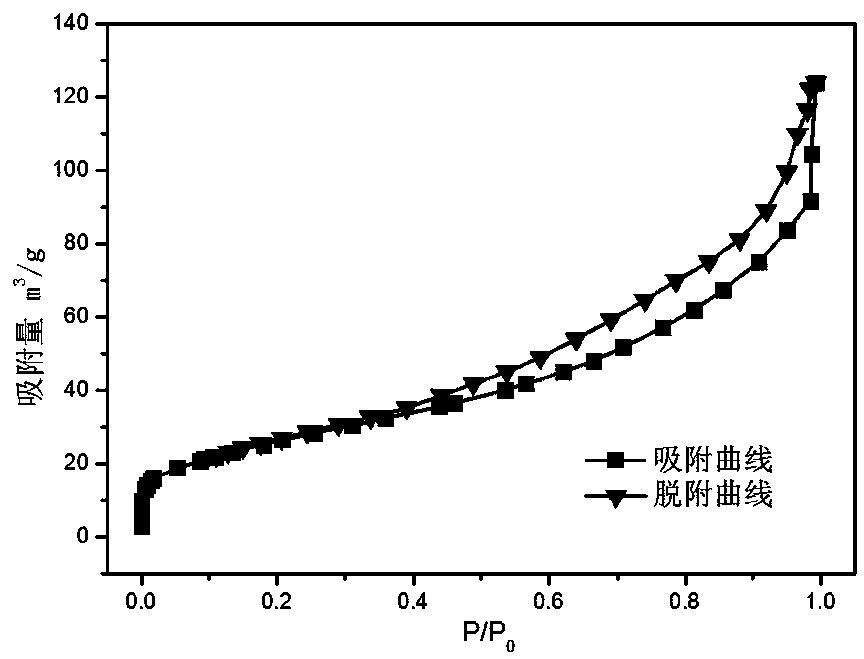
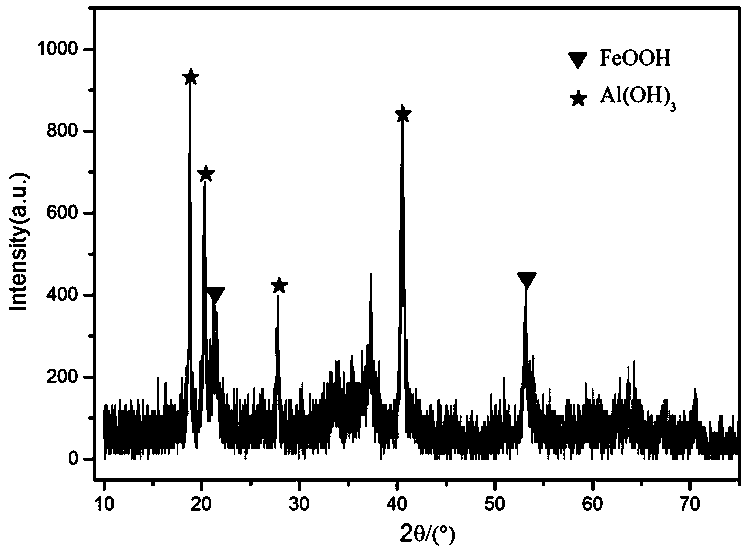
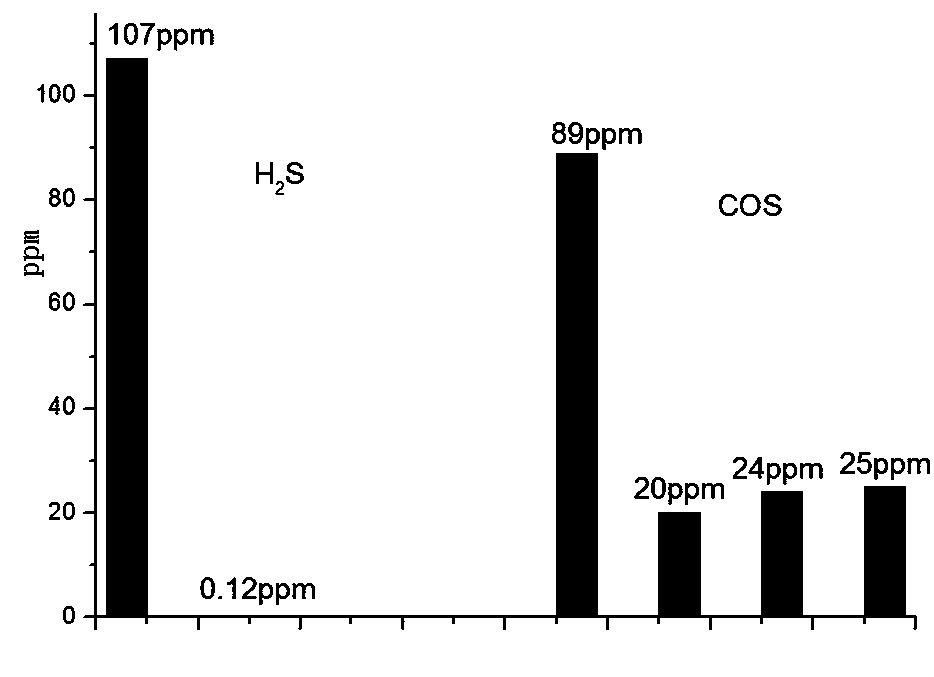
Image
Examples
Embodiment 1
[0026] The preparation process of bifunctional iron oxyhydroxide is as follows:
[0027] (1) Preparation of ferrous salt solution
[0028] Weigh 5.76g of ferrous nitrate, control the temperature at 25°C, dissolve it in a beaker, pour it into a 100ml volumetric flask, wash the beaker several times and introduce water into the volumetric flask, and titrate to 100ml to obtain a ferrous salt solution;
[0029] (2) Preparation of sodium metaaluminate solution
[0030] Weigh 1.66g and dissolve it in a beaker, introduce it into a 100ml volumetric flask, wash the beaker several times and import water into the volumetric flask, titrate to 100ml to obtain a sodium metaaluminate solution;
[0031] (3) Mix well
[0032] The ferrous salt solution is pumped into the sodium metaaluminate solution at a flow rate of 1ml / min through a peristaltic pump, the temperature is controlled at 25°C, magnetic stirring is performed while the ferrous salt is being pumped in, and air is introduced at a fl...
Embodiment 2
[0057] (1) Preparation of ferrous salt solution
[0058] Weigh 5.18g of ferrous nitrate, control the temperature at 30°C, dissolve it in a beaker, pour it into a 100ml volumetric flask, wash the beaker several times and introduce water into the volumetric flask, titrate to 100ml to obtain a ferrous salt solution;
[0059] (2) Preparation of sodium metaaluminate solution
[0060] Weigh 1.66g and dissolve it in a beaker, introduce it into a 100ml volumetric flask, wash the beaker several times and import water into the volumetric flask, titrate to 100ml to obtain a sodium metaaluminate solution;
[0061] (3) Mix well
[0062] Pump the ferrous salt solution into the sodium metaaluminate solution at a flow rate of 0.5ml / min through a peristaltic pump. The temperature is controlled at 30°C. Magnetic stirring is performed while the ferrous salt is being pumped in, and air is introduced, and the air flow rate is 20ml. / min, stirring for 5h to stop the reaction.
[0063] Reaction e...
Embodiment 3
[0072] (1) Preparation of ferrous salt solution
[0073] Weigh 3.77g of ferrous chloride, control the temperature at 28°C, dissolve it in a beaker, pour it into a 100ml volumetric flask, wash the beaker several times and import water into the volumetric flask, titrate to 100ml to obtain a ferrous salt solution;
[0074] (2) Preparation of sodium metaaluminate solution
[0075] Weigh 1.66g and dissolve it in a beaker, introduce it into a 100ml volumetric flask, wash the beaker several times and import water into the volumetric flask, titrate to 100ml to obtain a sodium metaaluminate solution;
[0076] (3) Mix well
[0077] Pump the ferrous salt solution into the sodium metaaluminate solution at a flow rate of 0.5ml / min through a peristaltic pump. The temperature is controlled at 30°C. Magnetic stirring is performed while the ferrous salt is being pumped in, and air is introduced, and the air flow rate is 40ml / min, stirring for 2h to stop the reaction.
[0078] Reaction equa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com