Mammalian cell line for protein production and library generation
A mammalian and cell line technology, applied in the field of protein libraries for directed evolution and protein engineering, can solve problems such as ineffective expression and screening of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0110] The present invention is further illustrated by the following examples and drawings, from which further embodiments and advantages can be obtained. These examples are intended to illustrate the invention without limiting its scope.
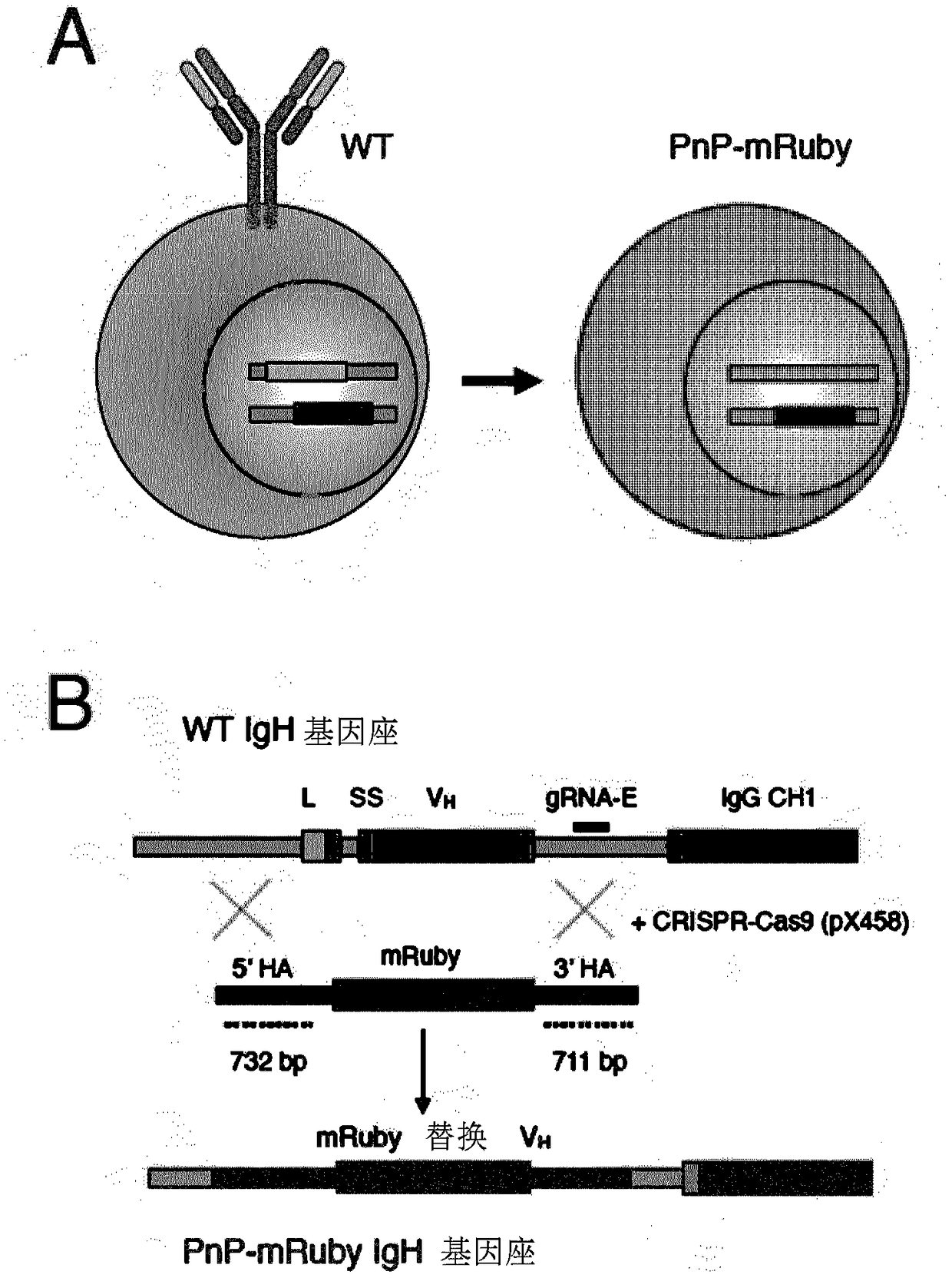
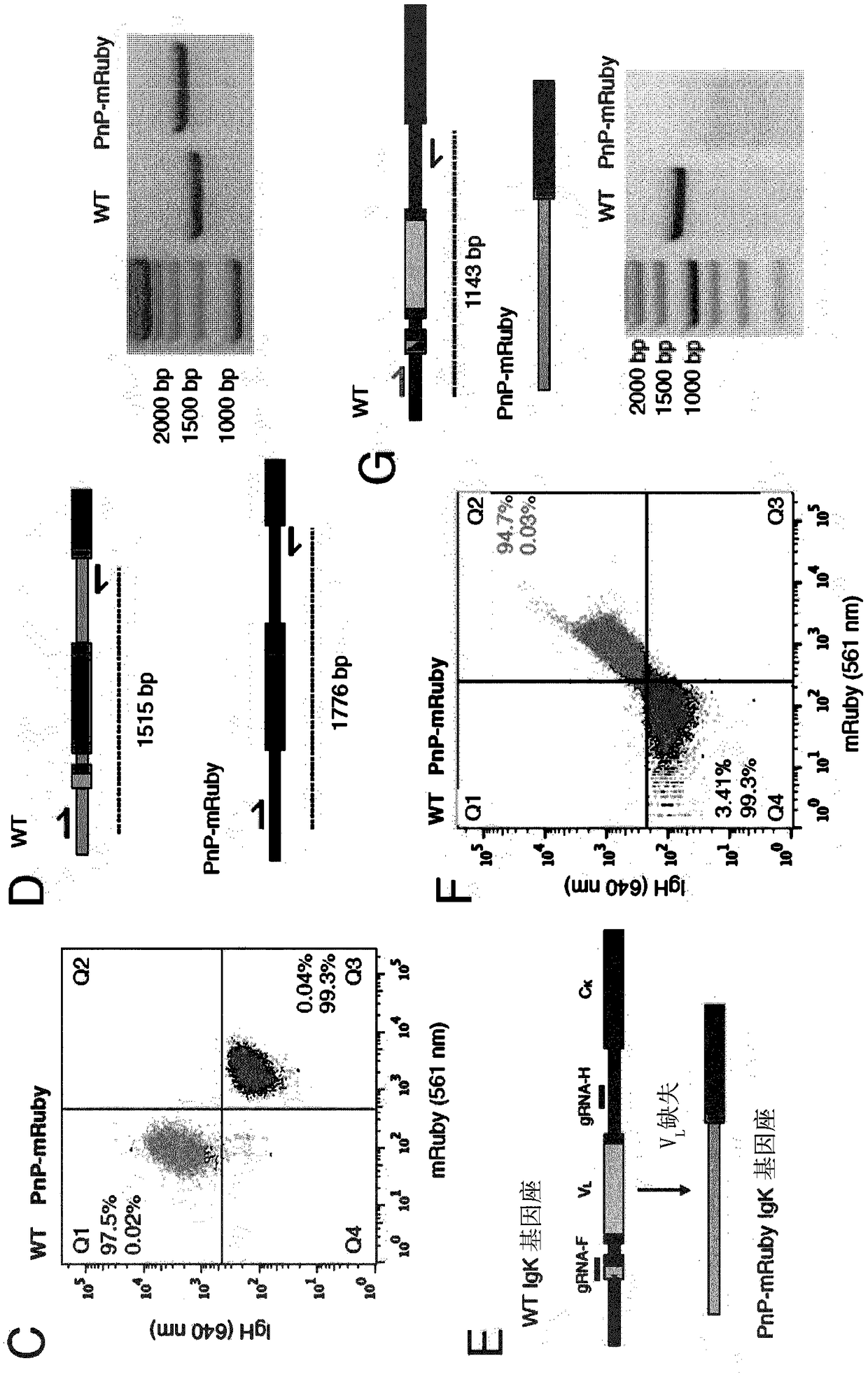
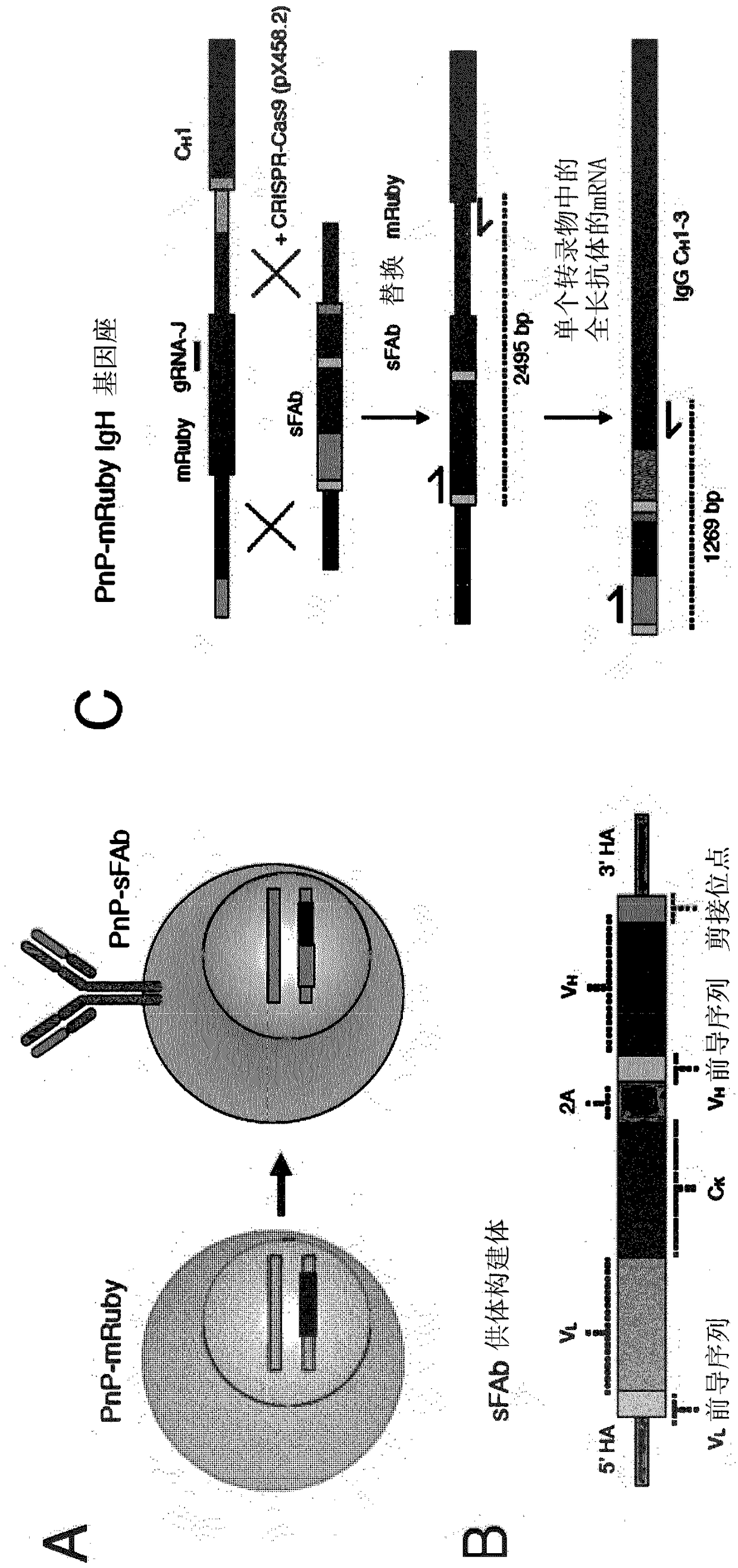
[0111] To carry out the present invention, the inventors have generated a plug-and-(dis)play (PnP) mammalian cell line. The inventors used mouse B lymphocytes (hybridoma cells), which serve as production factories for antibody proteins. PnP cell lines consist of the following components:
[0112] 1) Endogenous V in the IgH locus H The gene was replaced by a fluorescent protein (mRuby, originally called mRuby2, derived from Addgene.org plasmid #:40260 (Lam et al., Nat Methods 2012, 9:1005-1012)). This was achieved by transfecting WT hybridoma cells with a CRISPR-Cas9 plasmid (pX458) (Addgene.org plasmid #: 48138) (Cong et al., Science 2013, 339:819-823) with a guide RNA (gRNA), so The guide RNA targets V H and the intron between the IgG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com