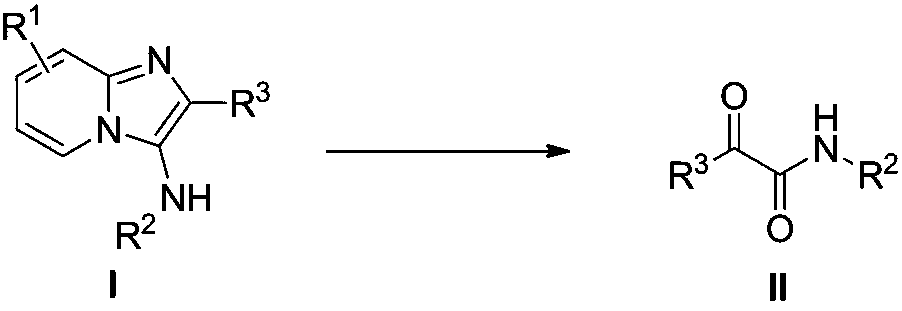
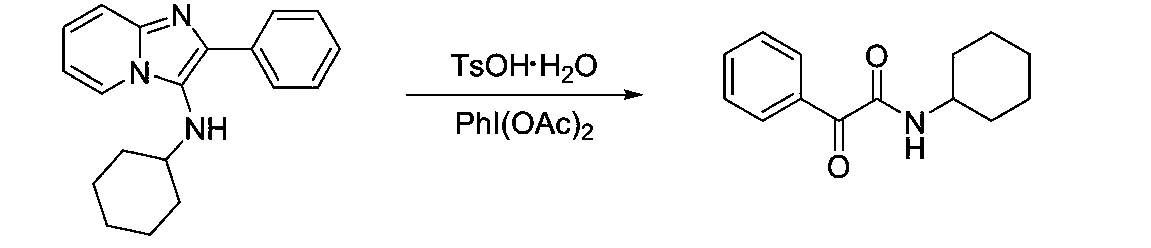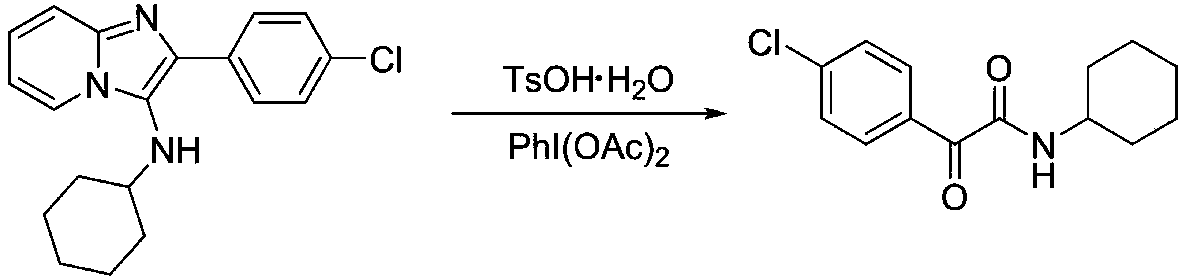Preparation method of alpha-carbonyl amide derivatives
A technology of carbonyl amides and derivatives, applied in the field of organic synthesis, to achieve excellent yields, high reaction yields, and improved atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of N-cyclohexyl-2-oxo-2-phenylacetamide
[0021]
[0022] 1mmol N-cyclohexyl-2-phenylimidazol[1,2-a]pyridin-3-amine, 3mmol TsOH·H 2 O was added to a 10mL reaction tube with a stirring bar, 2.5mL of dichloroethane was added, and stirred. After stirring for 5 minutes, 1 mmol of iodobenzene acetate was added, stirred at room temperature, TLC followed the reaction, and the reaction was stopped after 1 hour. Analysis (petroleum ether: ethyl acetate = 5:1), precipitation, the target compound was obtained, the yield was 99.1%, light yellow solid; m.p.91-92°C; 1 H NMR (400MHz, Chloroform) δ8.33(d, J=7.3Hz, 2H), 7.62(t, J=7.4Hz, 1H), 7.47(t, J=7.7Hz, 2H), 6.97(s, 1H ),4.05–3.71(m,1H),2.07–1.93(m,2H),1.81–1.72(m,2H),1.70–1.63(m,1H),1.49–1.36(m,2H),1.33–1.21 (m,3H). 13 C NMR (101MHz, Chloroform) δ188.15, 160.86, 134.31, 133.47, 131.23, 128.46, 48.49, 32.73, 25.43, 24.76. HR-MS (ESI): Calculated for C 14 h 16 ClNO 2 [M+H] + :231.12593,found:231.1...
Embodiment 2
[0023] Embodiment 2: Preparation of 2-(4-chlorophenyl)-N-cyclohexyl-2-oxo-acetamide
[0024]
[0025]1mmol 2-(4-chlorophenyl)-N-cyclohexaneimidazol[1,2-a]pyridin-3-amine, 3mmol TsOH·H 2 O was added to a 10mL reaction tube with a stirring bar, 2.5mL of dichloroethane was added, and stirred. After stirring for 5 minutes, 1 mmol of iodobenzene acetate was added, stirred at room temperature, TLC followed the reaction, and the reaction was stopped after 1 hour. Analysis (petroleum ether: ethyl acetate = 5:1), precipitation, the target compound was obtained with a yield of 94.8%; white solid; m.p.90-91°C; 1 H NMR (500MHz, Chloroform-D) δ8.32 (d, J = 8.6Hz, 2H), 7.43 (d, J = 8.7Hz, 2H), 6.99 (s, 1H), 3.82 (m, 1H), 2.03 –1.92(m,2H),1.79–1.71(m,2H),1.73–1.56(m,1H),1.45–1.34(m,2H),1.24(m,3H). 13 C NMR (126MHz, DMSO-D6) δ189.91, 164.37, 139.99, 132.20, 132.02, 129.75, 48.31, 32.54, 25.57, 25.03. HR-MS (ESI): Calculated for C 14 h 16 ClNO 2 [M+H] + :266.09423,found:266.09384.
Embodiment 3
[0026] Embodiment 3: Preparation of N-cyclohexyl-2-(3-nitrophenyl)-2-oxo-acetamide
[0027]
[0028] 1mmol 2-(3-nitrophenyl)-N-cyclohexaneimidazol[1,2-a]pyridin-3-amine, 3mmol TsOH·H 2 O was added to a 10mL reaction tube with a stirring bar, 2.5mL of dichloroethane was added, and stirred. After stirring for 5 minutes, 1 mmol of iodobenzene acetate was added, stirred at room temperature, TLC followed the reaction, and the reaction was stopped after 1 hour. Analysis (petroleum ether: ethyl acetate = 5:1), precipitation, the target compound was obtained, the yield was 97.4%, white solid; m.p.91-92°C; 1 H NMR (500MHz, Chloroform-D) δ9.13–9.11(t,1H),8.66(tt,J=7.8,1.3Hz,1H),8.42(m,1H),7.65(t,J=8.0Hz, 1H),7.01(s,1H),1.45(s,9H). 13 C NMR (126MHz, Chloroform-D) δ186.25, 159.94, 148.21, 136.96, 134.72, 129.66, 128.21, 126.16, 52.07, 28.39. HR-MS (ESI): Calculated for C 12 h 14 N 2 o 4 [M+H] + :251.10.263,found:251.10222.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com