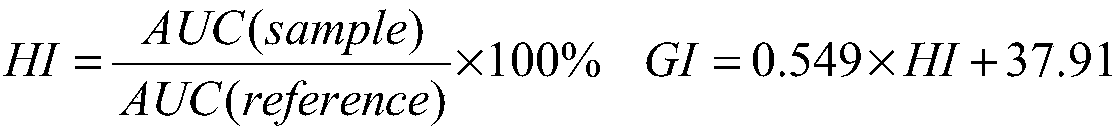Preparation method of low-blood-glucose-index resistant starch type recombination rice
A low-glycemic index and resistant starch technology, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of easy production of harmful substances in the preparation process, complex preparation methods, and product effects. Bad question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1: Preparation of low glycemic index resistant starch
[0115] details as follows:
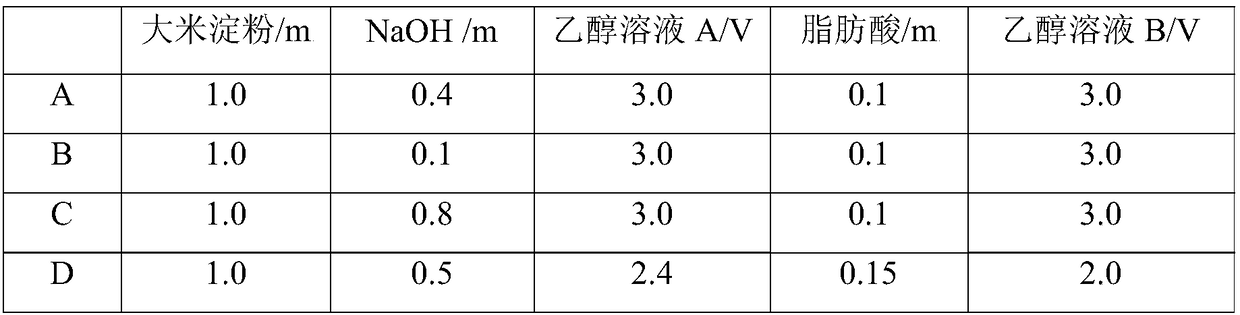
[0116] (1) get each raw material with the formula of table 1;
[0117] (2) Weigh rice starch and disperse it in 55% (v / v) ethanol solution A, prepare 20% (w / v) starch milk, keep it warm in a constant temperature water bath shaker at 45°C, and at the same time, use 2mL / min Add 1.0mol / L NaOH solution at the rate of 1.0mol / L, and fully react for 25min;
[0118] (3) Fatty acid is dissolved in the ethanol solution B of 65% (v / v), until the ratio of starch quality, NaOH quality, ethanol solution A volume, fatty acid quality, and ethanol volume solution B in the reaction solution is carried out according to Table 1 Adjust, then preheat in a constant temperature water bath at 50°C for 5 minutes, then add it to the starch milk system at a rate of 0.4mL / min, fully react for 75 minutes, and then add 1.0mol / L HCl solution to adjust the pH to neutral;
[0119] (4) After completing the ...
Embodiment 2
[0130] Embodiment 2: Preparation of low glycemic index resistant starch
[0131] details as follows:
[0132] (1) Weigh rice starch and disperse it in 55% (v / v) ethanol solution A, prepare 20% (w / v) starch milk, keep it warm in a constant temperature water bath shaker at 45°C, and at the same time use 2mL / min Add 1.0mol / L NaOH solution at a rate of 1.0mol / L until the ratio of starch mass, NaOH mass and ethanol volume in reaction solution A is 1.0:0.4:3.0, and fully react for 25min;
[0133] (2) Dissolve fatty acid in 65% (v / v) ethanol solution B until the ratio of starch mass, fatty acid mass, NaOH mass and ethanol volume in reaction solution B is 1.0:0.1:0.4:3.0, at 50°C After preheating in a constant temperature water bath for 5 minutes, add it to the starch milk system at a rate of 0.4 mL / min, fully react for 75 minutes, and then add 1.0 mol / L HCl solution to adjust the pH to neutral;
[0134] (3) The specific operating conditions of low temperature complexation and cooli...
Embodiment 3
[0145] Embodiment 3: Preparation of low glycemic index recombinant rice
[0146] details as follows:
[0147] (1) Take each raw material with the formula in Table 5 (the low blood sugar resistant starch here is the low blood sugar resistant starch obtained in Group A in Example 2);
[0148] (2) After fully mixing the raw materials with a stirrer, add distilled water equivalent to 35% of the mass of the material, add water to temper, and mix again to obtain a mixture;
[0149] (3) After quenching and tempering is completed, the mixture is fed into the twin-screw extruder, and the solid feeding rate is 3kg / h before the start of extrusion. 80°C, 90°C, 100°C, the screw speed is 110rpm, the diameter of the die head at the discharge port of the extruder is 6mm, and the cutting machine speed at the discharge port is 250rpm;
[0150] (4) Carry out extruding granulation by twin-screw extruder, and cut with cutter at die head, obtain granule or particle size be the spherical or rod-sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com