no 2 -Alkenyl benzotriazole derivatives and synthesis method thereof
A technology of alkenyl benzotriazoles and synthesis methods, which is applied in the field of N2-alkenyl benzotriazole derivatives and their synthesis, can solve the problems such as the reduction of benzotriazole aromaticity, and achieve simple and mild reaction conditions , The synthesis method is simple and cheap, and the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
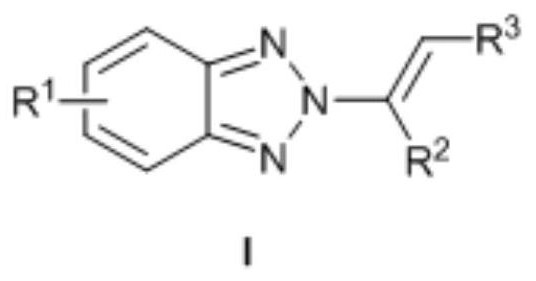
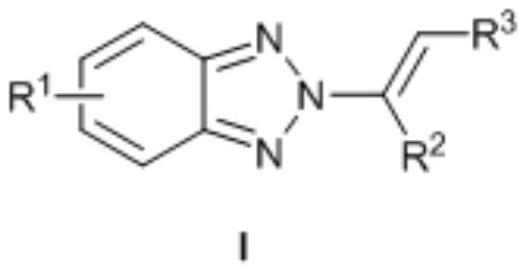
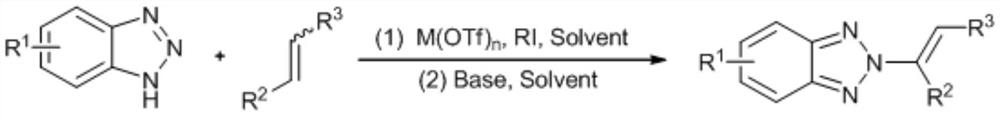
[0023] N as above 2 - the synthetic method of alkenyl benzotriazole derivatives, concrete steps are as follows: in solvent I, in catalyst M (OTf) n Under the catalysis of benzotriazole, iodine source and olefin react at room temperature for 0.5-5h, after concentration, add solvent II and base, react at 50-70°C for 8-15h, and get the target after concentration and column chromatography compound.
[0024] Solvent I is chloroform, dichloromethane, dichloroethane, ethyl acetate or 1,4-dioxane.
[0025] Catalyst M(OTf) n is Zn(OTf) 2 , Cu(OTf) 2 or Al(OTf) 3 .
[0026] Catalyst M(OTf) n The number of moles is 2-10% of the number of moles of benzotriazole.
[0027] The base is potassium tert-butoxide, sodium tert-butoxide, sodium hydride, potassium carbonate or sodium carbonate.
[0028] The molar number of base is 2-5 times of that of benzotriazole.
[0029] The molar ratio of benzotriazole, iodine source and alkene is 1:(1-2):(1-3).
[0030] The iodine source is N-iodos...
Embodiment 1
[0043] With chloroform as solvent, 0.05mol Zn(OTf) 2 As a catalyst, 1.0mol benzotriazole, 1.5mol N-iodosuccinimide and 2.0mol styrene were reacted at room temperature for 1 hour, then concentrated, using methanol as solvent, 5.0mol potassium carbonate as base, 60°C After reacting for 12h, the target compound A can be obtained through concentration and column chromatography, and the yield is 81%, N 2 - 95% selectivity.
[0044] 1 H NMR (400MHz, CDCl 3 ):δ7.94-7.89(m,2H),7.47-7.41(m,7H),6.21(s,1H),5.67(s,1H); 13 C NMR (100MHz, CDCl 3 ): δ147.2, 144.7, 134.6, 129.6, 128.5, 128.3, 127.3, 118.5, 111.1.
Embodiment 2
[0046] Its specific synthetic steps refer to Example 1, and the olefin is 4-tert-butylstyrene.
[0047] B yield is 90%, N 2 - 99% selectivity.
[0048] 1 H NMR (400MHz, CDCl 3 ):δ7.95-7.90(m,2H),7.46(d,J=8.5Hz,2H),7.43-7.41(m,2H),7.39(d,J=8.4Hz,2H),6.15(s, 1H), 5.65(s, 1H); 13 C NMR (100MHz, CDCl 3 ): δ152.7, 147.1, 144.7, 131.7, 127.9, 127.2, 125.5, 118.5, 110.6, 34.8, 31.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com