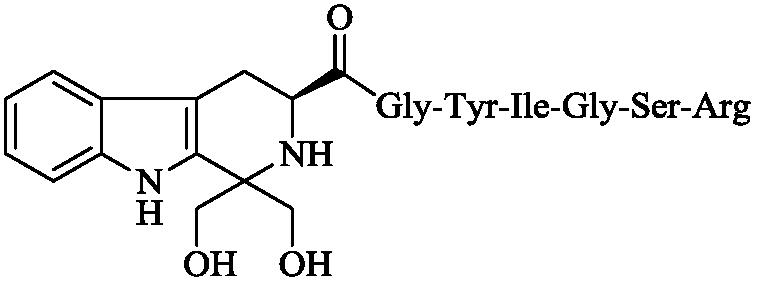
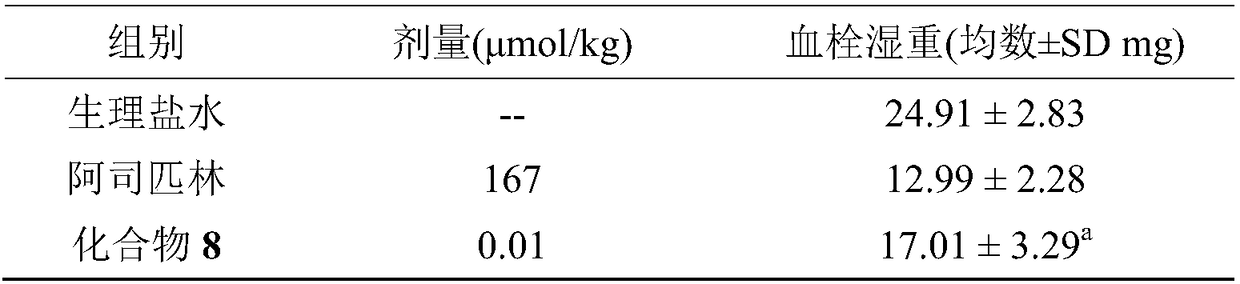
Synthesis, activity and application for 1,1 dimethylol-tetrahydro-beta-carboline-3-formyl-GYIGSR
A technology of dimethylol and dihydroxyacetone, applied in 1,1-dimethylol-tetrahydro-β-carboline-3-formyl-GYIGSR, its synthesis, activity and application fields, can solve the problem of vein Ineffective thrombosis, bleeding side effects, no anti-venous thrombosis activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
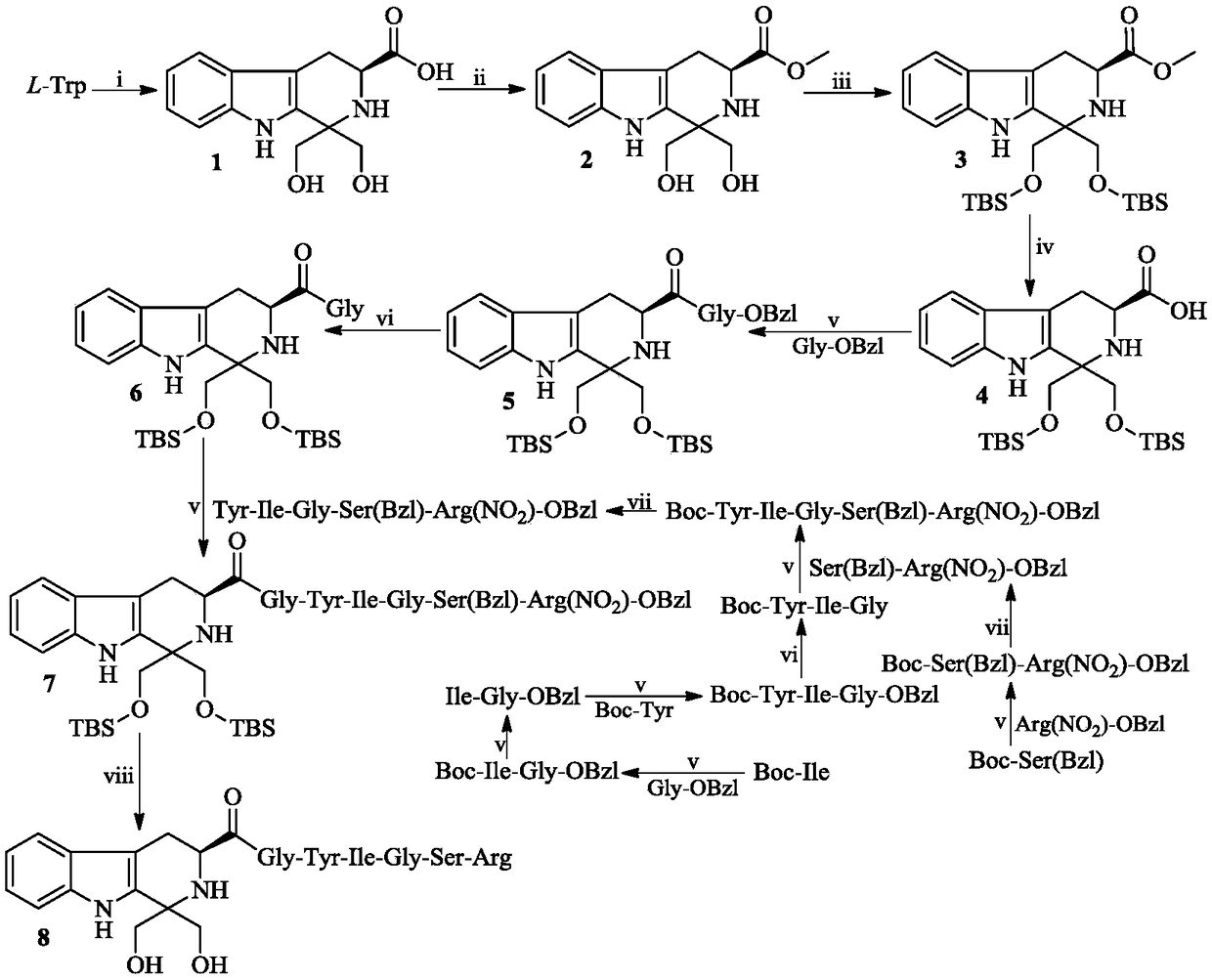
[0021] Example 1 Preparation of (3S)-1,1-dimethylol-tetrahydro-β-carboline-3-carboxylic acid (1)
[0022] Suspend 6.12 g (30 mmol) of L-tryptophan in 100 mL of distilled water. Under an ice bath, slowly add concentrated sulfuric acid dropwise until the L-tryptophan is completely dissolved. Add 3.24 g (36 mmol) of 1,3-dihydroxyacetone to the solution, and react at room temperature for 72 hours. TLC (ethyl acetate / water / glacial acetic acid, 10 / 1 / 2) showed that the reaction was complete. Filtration and the filter cake was rinsed with ice water to afford 6.13 g (74%) of the title compound as a yellow powder.
Embodiment 2
[0023] Example 2 Preparation of (3S)-1,1-dimethylol-tetrahydro-β-carboline-3-carboxylic acid methyl ester (2)
[0024] Slowly add 5.2 mL of thionyl chloride dropwise to 55 mL of methanol in an ice-salt bath, and stir for 30 minutes. 5.52 g (20 mmol) of (3S)-1,1-dimethylol-tetrahydro-β-carboline-3-carboxylic acid (1) was added to the solution, and stirred until completely dissolved. Stir at room temperature for 12 hours. TLC (dichloromethane / methanol, 20:1) showed the reaction was complete. Concentrate under reduced pressure, and the residue is triturated with ethyl acetate to obtain a brown-yellow solid. The solid was dissolved with 200 mL of ethyl acetate, and the resulting solution was successively washed with saturated NaHCO 3 Wash with aqueous solution (30 mL×3) and saturated NaCl aqueous solution (30 mL×3), and dry over anhydrous sodium sulfate for 12 hours. Filtration and concentration of the filtrate under reduced pressure afforded 5.34 g (92%) of the title compound...
Embodiment 3
[0025] Example 3 Preparation of (3S)-1,1-bis(tert-butyldimethylsilyloxy)methyl-tetrahydro-β-carboline-3-carboxylic acid methyl ester (3)
[0026] Mix 5.22g (18mmol) (3S)-1,1-dimethylol-tetrahydro-β-carboline-3-carboxylic acid methyl ester (2) with 50mL of anhydrous N,N-dimethylformamide ( DMF) was stirred until completely dissolved. Under ice cooling, add 4.4 g (64.8 mmol) imidazole to the solution and stir until completely dissolved. Add 8.15 g of tert-butyldimethylsilyl chloride (TBDMSCl) to the solution, and stir at room temperature for 12 hours. TLC (petroleum ether / ethyl acetate, 20 / 1) showed that the reaction was complete. Under ice-cooling, 350 mL of saturated NaCl aqueous solution was first added to the solution, and then extracted three times with ethyl acetate. The ethyl acetate layer was sequentially washed with saturated NaHCO 3 Wash with aqueous solution (40mL×3) and saturated NaCl aqueous solution (40mL×3), and dry over anhydrous sodium sulfate for 12 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com