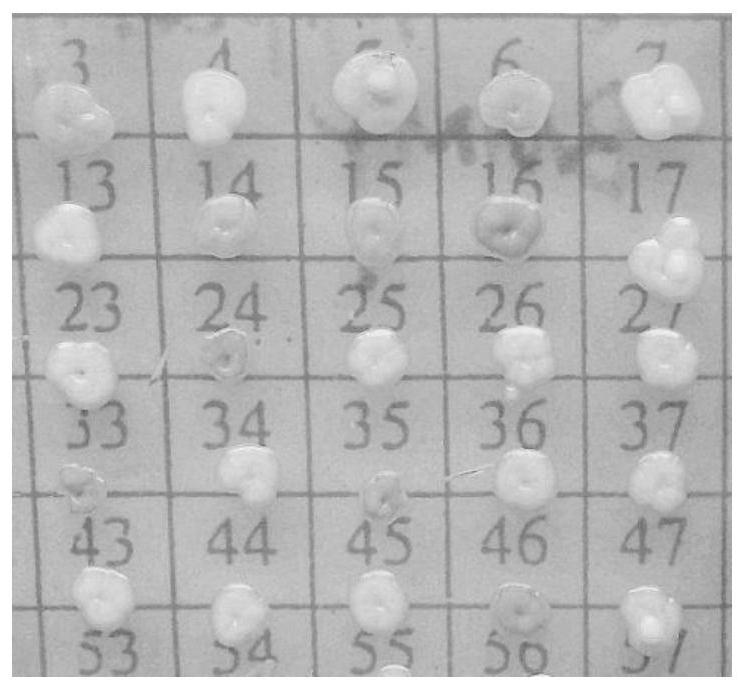
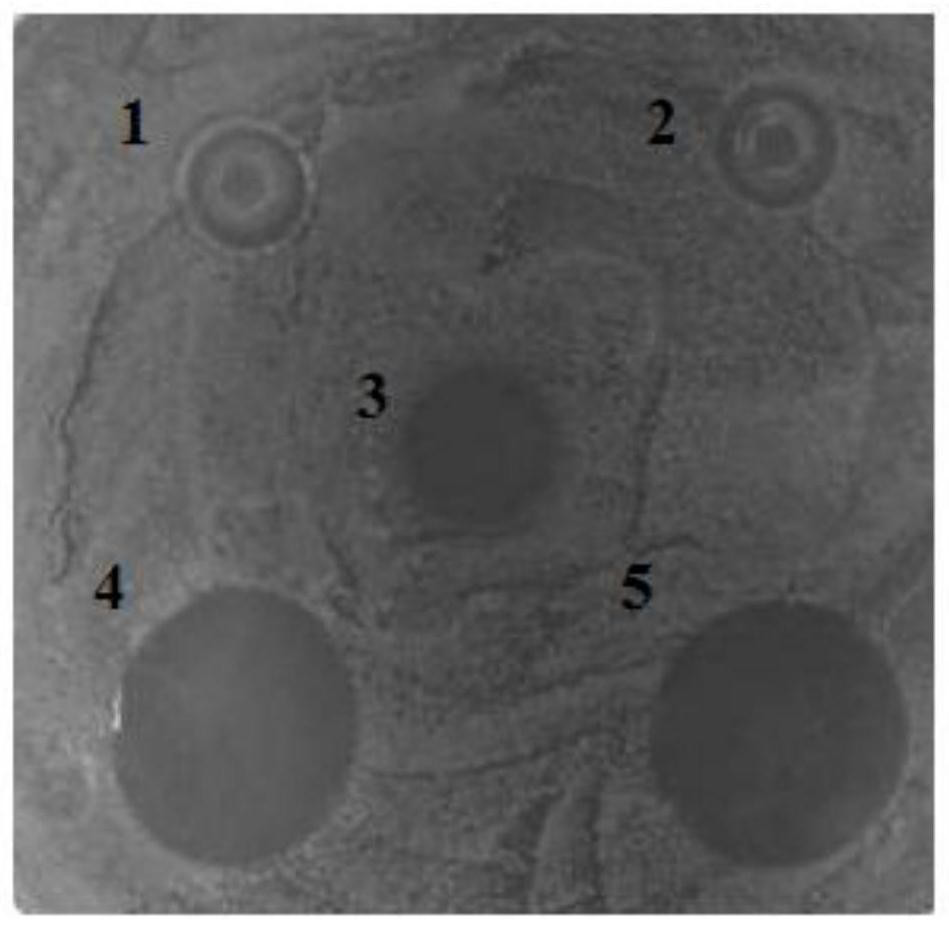
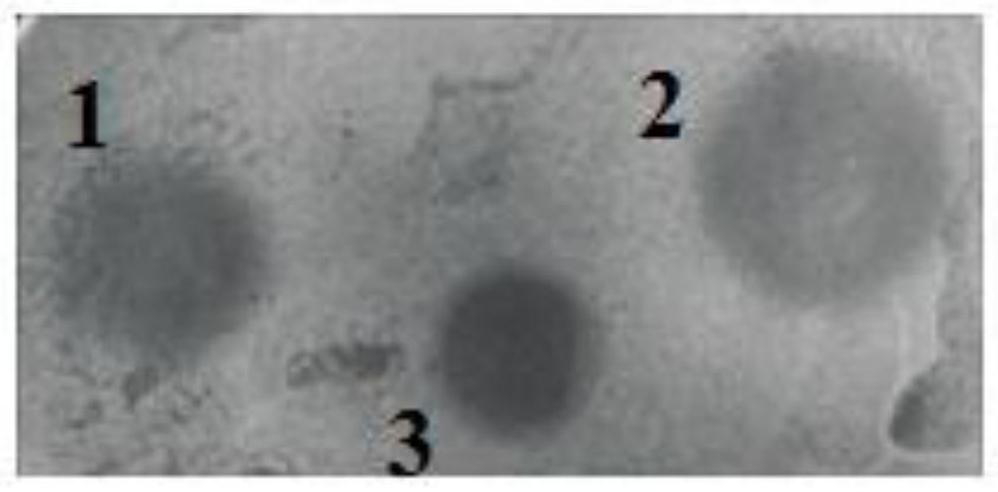
A visual antimicrobial peptide fusion protein and its preparation method and application
An antibacterial peptide and recombinant protein technology, applied in the field of group engineering, can solve the problems of consuming a lot of manpower and material resources, protease attack, affecting the expression efficiency, etc., to achieve good application prospects, increase expression, and reduce the effect of possibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1, red fluorescent protein mApple gene acquisition and sequence analysis
[0077] According to the known sequence of the gene mApple, using the codon preference of Pichia pastoris, the sequence was optimized and transformed without changing its amino acid sequence, and a his purification tag was added at the 5' end, and the sequence was sent to the company for synthesis. The red fluorescent protein mApple contains 236 amino acids, plus 6 histidines, a total of 242 amino acids, and the theoretical molecular weight of the mature protein is 28kDa. The amino acid sequence of the red fluorescent recombinant protein his-mApple with his purification tag added is shown in the sequence table Shown in SEQ ID NO.1;
[0078] The nucleotide sequence of the coding gene of the red fluorescent recombinant protein his-mApple that is modified by optimizing the codon bias of Pichia pastoris and added with the his purification tag is shown in SEQ ID NO.2 in the sequence listing....
Embodiment 2
[0085] Example 2, the gene of honeybee antimicrobial peptide AP precursor and the synthesis of DP dipeptide acid cleavage site
[0086] According to the known sequence of honeybee antimicrobial peptide AP gene, according to the codon preference of Pichia pastoris, the nucleotide sequence was optimized without changing its amino acid sequence, and the gene coding for DP dipeptide was added at its 5' end Sequence: GACCCA, send the company to synthesize the nucleotide sequence. The honeybee antimicrobial peptide AP has a total of 18 amino acids, and the theoretical molecular weight of the mature protein is 2.2kDa. The amino acid sequence of the antimicrobial peptide AP is shown in SEQ ID NO.3;
[0087] The nucleotide sequence encoding the honeybee antimicrobial peptide AP modified by optimizing the codon preference of Pichia pastoris is shown in SEQ ID NO.4 in the sequence listing.
[0088] The nucleotide sequence encoding the honeybee antimicrobial peptide AP of the gene coding...
Embodiment 3
[0093] Embodiment 3, the acquisition of the fusion gene his-mApple-DP-AP
[0094] The PCR product A obtained in Example 1 and the PCR product obtained in Example 2 were used as a common template, and mApple-F1 and AP-R were used as primers for PCR amplification.
[0095]The PCR product B obtained in Example 1 and the PCR product obtained in Example 2 were used as a common template, and mApple-F2 and AP-R were used as primers for PCR amplification.
[0096] The PCR reaction parameters were: 95°C for 5 min; 32 cycles of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 90 sec; 72°C for 10 min. PCR Product Purification Kit to purify PCR products.
[0097] The sequencing results of the above PCR amplification products show that the two nucleic acid fragments obtained by the above PCR amplification respectively include XhoI, Not I double restriction sites and EcoR I, Not I double restriction sites, respectively named his-mApple-DP -AP-1 and his-mApple-DP-AP-2, the middle of the two ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com