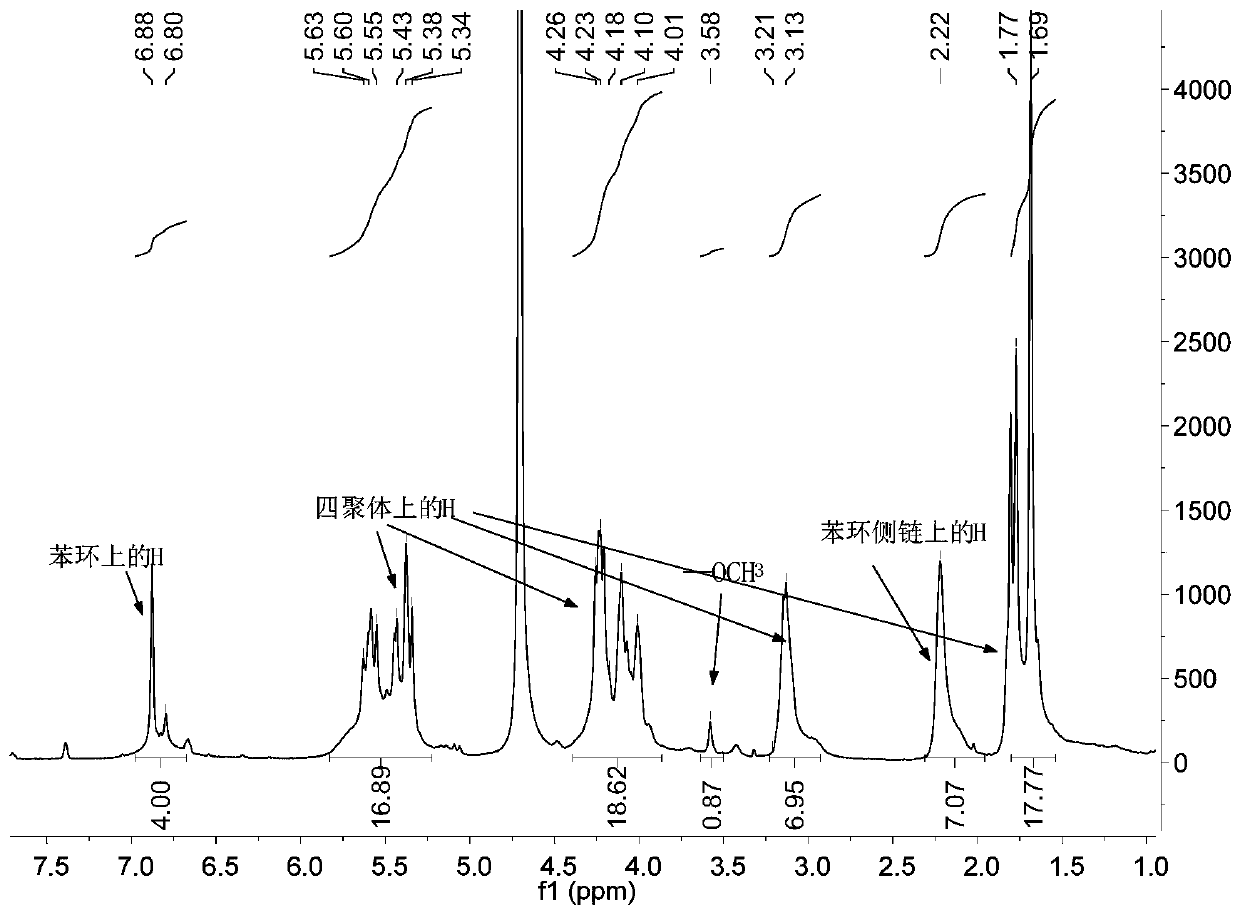
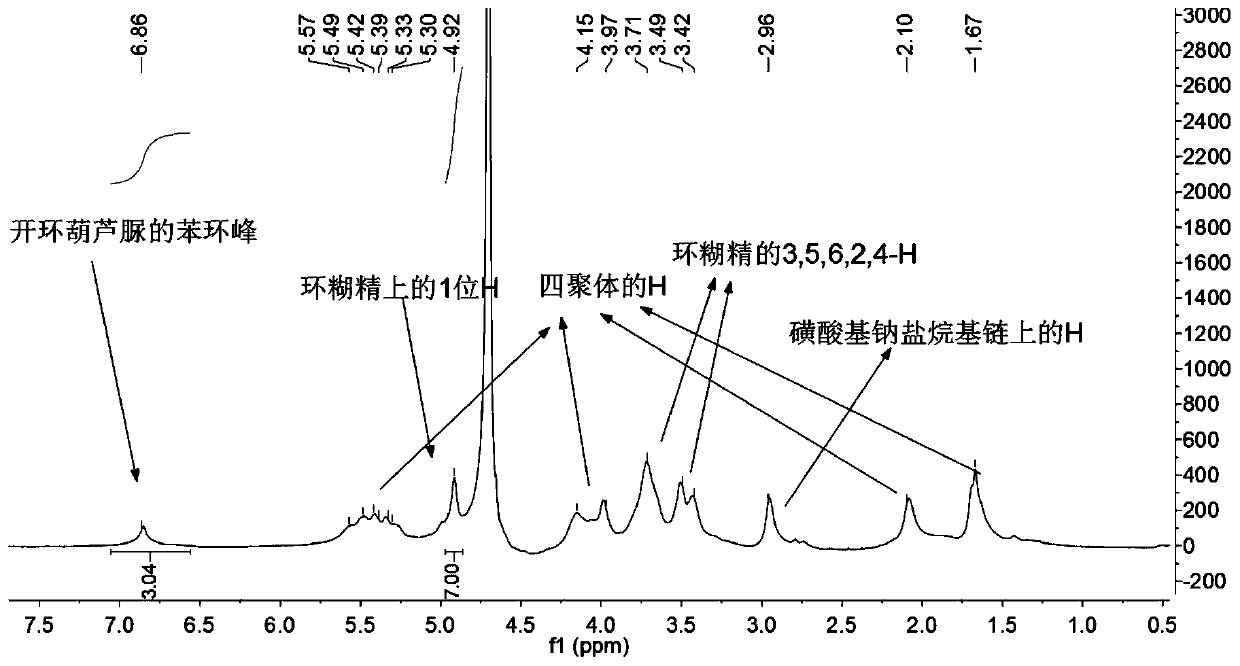
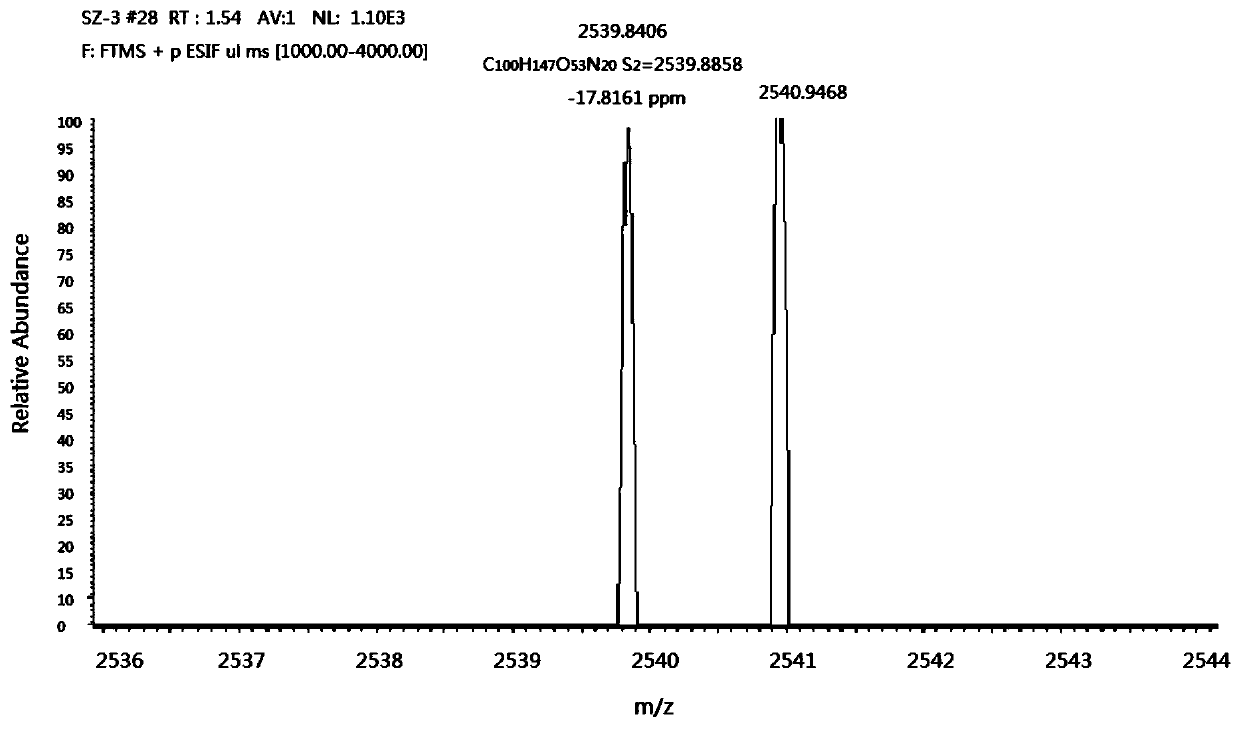
Linker of amino-modified cyclodextrin bonded to open-ring cucurbituril and its preparation method and application
A technology of cucurbituril and cyclodextrin, which is applied in the field of linkages of amine-modified cyclodextrins bonded to open-ring cucurbituril and its preparation, which can solve the problem of high drug size and cavity matching, drug water Poor solubility, lower bioavailability and other problems, to achieve the effect of improving water solubility and bioavailability, increasing solubility, and increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Synthetic preparation of tetramer and sulfonic acid sodium salt benzene ring side arm and
[0041] Carry out with reference to the method in literature Ma D, et al.Nature Chemistry, 2012,4(6):503:
[0042] (1) Synthesis of dimer: mix glycoluril (500g, 3.51mol) and paraformaldehyde (105g, 3.51mol) evenly, add to HCl solution (8M, 700mL), and heat the reaction solution at 50°C for 48h; After the reaction, the reaction solution was cooled and vacuum filtered to obtain the crude product; the crude product was washed with water (500 mL), and then recrystallized with TFA (1.5 L) to obtain a dimer as a white solid (334 g, 62%) ;
[0043]
[0044] (2) Synthesis of tetramer: dimer (304g, 1.20mol) was added to anhydrous MeSO 3 In H (600mL), after the dimer is completely dissolved, the solution becomes transparent, then etherified methyl glycoluril (84g, 0.27mol) is added; the reaction solution is reacted at 50°C for 3h; The solution was poured into water (6.0L...
Embodiment 2
[0048] Embodiment 2: the synthesis of carboxyl-containing benzene ring side arm
[0049] 3g (0.024mol) of p-methoxyphenol and 3mL of methyl bromopropionate, add some N,N-dimethylformamide to help dissolve, stir well and add 4g (0.029mol) of K 2 CO 3 , reacted for 20-24 hours to generate p-methoxyphenylpropionyl methyl ester; after that, add 3g (0.125mol) LiOH, 10mL H 2 O and 10 mL of tetrahydrofuran were used to hydrolyze the ester; the reaction finally produced 4.5 g of carboxyl-containing side arms, with a yield of 89.3%.
[0050]
Embodiment 3
[0051] Embodiment 3: the synthesis of ring-opening cucurbituril containing carboxylic acid group
[0052] Fully dissolve 1.8g (2.45mmol) of tetramer in 8mL of acetic anhydride and 8mL of trifluoroacetic acid, and mix thoroughly in an oil bath at 60°C to 80°C to completely dissolve the tetramer. After 5 to 10 minutes, Then add the benzene ring side arm of sodium sulfonate and the carboxyl-containing benzene ring side arm to continue the reaction for 3 to 4 hours; after the reaction, drop the reaction solution into a rapidly stirring organic solvent, such as acetone, methanol, etc. Extract it. Since there may be three different open-ring cucurbiturils in the generated product, extract and separate them according to their degree of hydrolysis; first, drop the reaction solution into rapidly stirring acetone, and after adding it completely, continue stirring for 0.5~ 1h, after suction filtration, put it into a vacuum drying oven for drying; dissolve the product with a certain amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com