Synthetic method of benzanilide
A technology of phenylbenzamide and synthesis method, which is applied in the field of N-phenylbenzamide synthesis, can solve the problems of difficult reaction control, complicated reaction, complicated operation, etc., and achieves thorough reaction, few reaction by-products, and high conversion rate. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
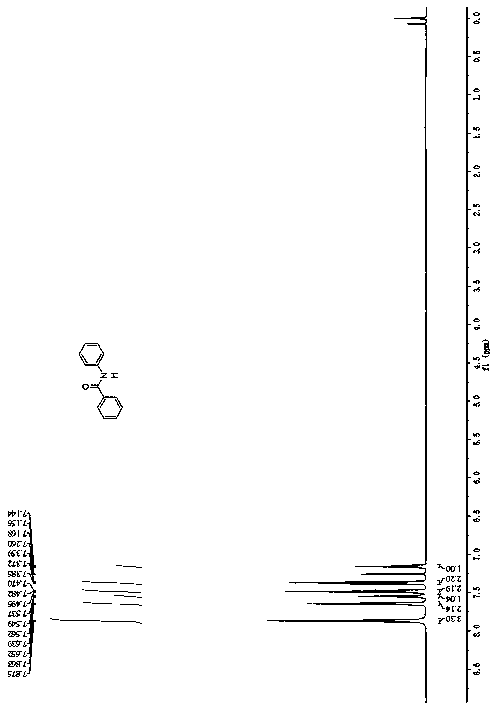
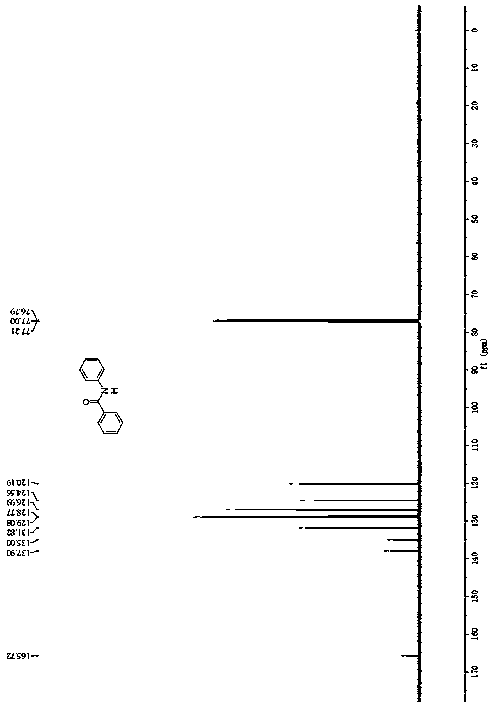
[0038] Example 1 A method for synthesizing N-phenylbenzamide, the method refers to dissolving the substrate α, N-diphenylnitrone and TBAF in a solvent, then adding alkali therein, and stirring and reacting at room temperature for 3 hours Finally, add water to quench the reaction to obtain a mixture; the mixture is extracted 3 times with ethyl acetate, the organic phases are combined, the organic phases are dried and concentrated under reduced pressure, and subjected to petroleum ether-ethyl acetate column chromatography to obtain a white solid N-phenylbenzamide, 57% yield.
[0039] Wherein: solvent refers to acetonitrile. The base refers to potassium hydroxide.
[0040] The mass volume ratio (g / mL) of α, N-diphenylnitrone to solvent is 1:140.
[0041] The mass ratio (g / g) of α, N-diphenylnitrone, TBAF, and base is 1:0.5:0.1.
[0042] The volume ratio (mL / mL) of petroleum ether to ethyl acetate in petroleum ether-ethyl acetate column chromatography is 10:1.
[0043] The con...
Embodiment 2
[0044] Example 2 A method for synthesizing N-phenylbenzamide, the method refers to dissolving the substrate α, N-diphenylnitrone and TBAF in a solvent, then adding alkali to it, and stirring and reacting at room temperature for 3 hours Finally, add water to quench the reaction to obtain a mixture; the mixture is extracted 3 times with ethyl acetate, the organic phases are combined, the organic phases are dried and concentrated under reduced pressure, and subjected to petroleum ether-ethyl acetate column chromatography to obtain a white solid N-phenylbenzamide, yield 62%.
[0045] Wherein: solvent refers to acetonitrile. The base refers to potassium hydroxide.
[0046] The mass volume ratio (g / mL) of α, N-diphenylnitrone to solvent is 1:140.
[0047] The mass ratio (g / g) of α, N-diphenylnitrone, TBAF, and base is 1:1:0.1.
[0048] Petroleum ether-ethyl acetate column chromatography is the same as in Example 1.
[0049] The conditions for concentrating under reduced pressure...
Embodiment 3
[0050] Example 3 A method for synthesizing N-phenylbenzamide, the method refers to dissolving the substrate α, N-diphenylnitrone and TBAF in a solvent, then adding alkali to it, and stirring and reacting at room temperature for 3 hours Finally, add water to quench the reaction to obtain a mixture; the mixture is extracted 3 times with ethyl acetate, the organic phases are combined, the organic phases are dried and concentrated under reduced pressure, and subjected to petroleum ether-ethyl acetate column chromatography to obtain a white solid N-phenylbenzamide, 65% yield.
[0051] Wherein: solvent refers to acetonitrile. The base refers to potassium hydroxide.
[0052] The mass volume ratio (g / mL) of α, N-diphenylnitrone to solvent is 1:140.
[0053] The mass ratio (g / g) of α, N-diphenylnitrone, TBAF, and base is 1:1.2:0.1.
[0054] Petroleum ether-ethyl acetate column chromatography is the same as in Example 1.
[0055] The conditions for concentrating under reduced pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com