A local anesthetic preparation for external use and a method for improving the stability of the local anesthetic preparation
A technology for local anesthesia and external preparations, which is applied in the field of local anesthetic preparations and amide local anesthetics. It can solve the problems of reducing drug efficacy, drug safety threats, and waste of raw materials for preparations, so as to improve stability and prolong drug storage. and shelf life, the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Product name: Debaian
[0027] Drug Name: Flurbiprofen Babu Ointment
[0028] Packaging material: Packaging material 5 paper / thermoplastic polyester / / aluminum / vinyl (PAPER / PET / AL / PE, Fujimori Industry Co., Ltd.)
[0029] Table 2 stability test:
[0030] serial number Influencing factors Dibaian (preparation content%) 1 Freeze at -30°C for 5 days 105.5% 2 Freeze at -30°C for 10 days 105.0% 3 Freeze at -30°C for 12 days 104.3% 4 40℃ high temperature for 10 days 105.2% 5 40°C high temperature for 12 days 104.3% 6 60℃ high temperature for 3 days 105.3% 7 60℃ high temperature for 5 days 105.1% 8 60℃ high temperature for 10 days 104.7% 9 60℃ high temperature for 12 days 104.5% 10 60℃ high temperature for 30 days 103.4% 11 5 days of light 105.1% 12 10 days of light 104.9% 13 92.5% high humidity for 5 days 104.5%
[0031] The results show that flurbiprofen uses paper / the...
Embodiment 2
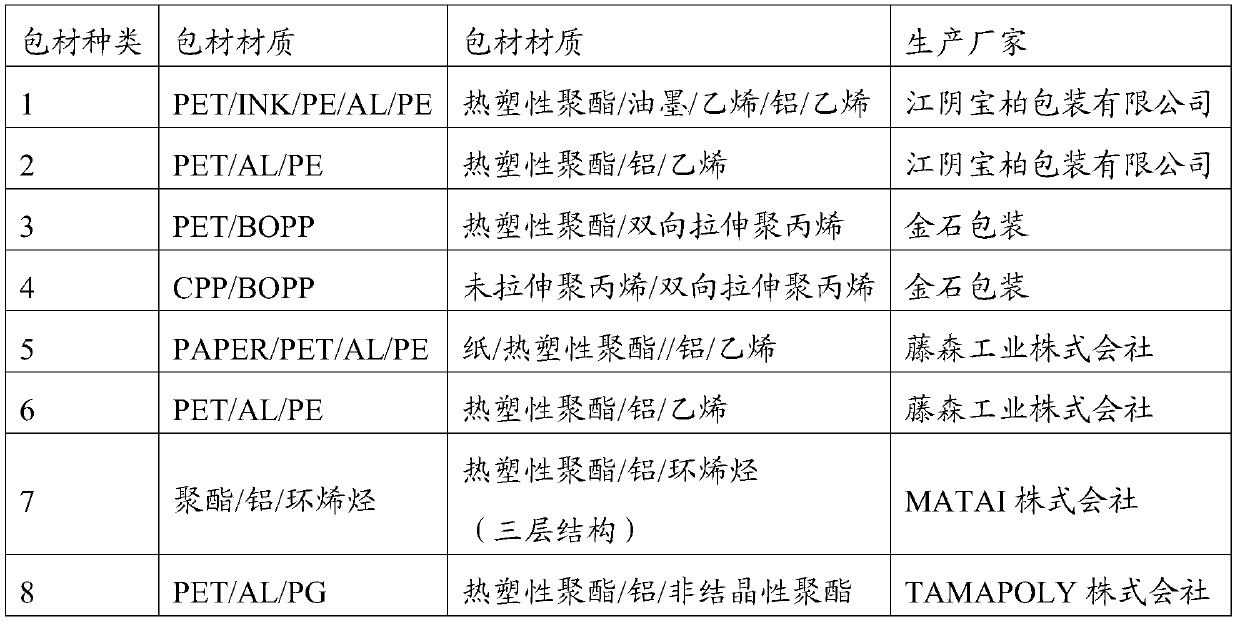
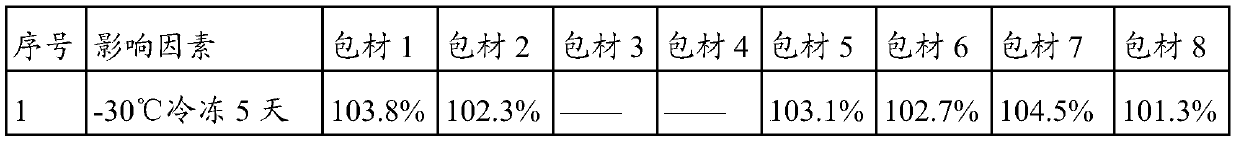
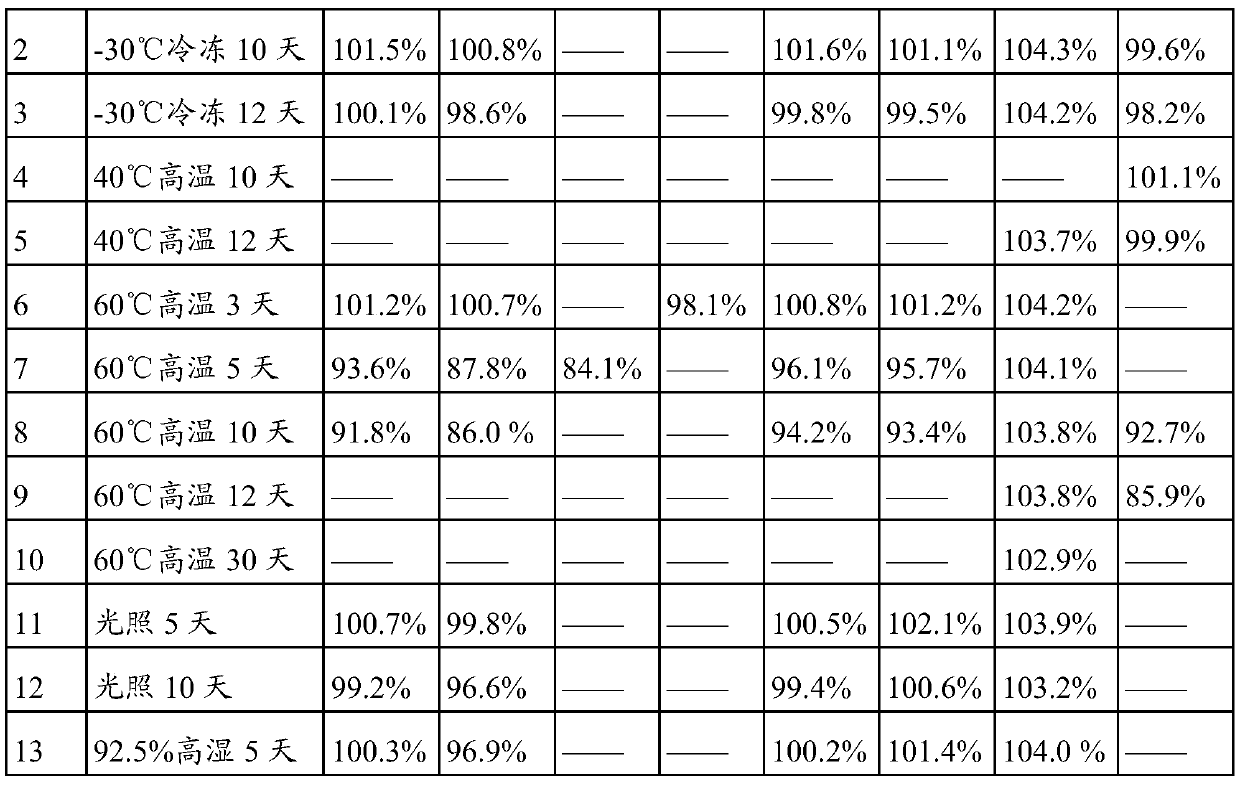
[0033] The raw material of lidocaine is unstable and has a certain sublimation property, so the packaging material of lidocaine patch needs better insulation and low adsorption to the main drug. In the present invention, the prepared lidocaine patches are packaged with different packaging materials. The packaging materials are shown in Table 3, and they are set out under different influencing factors. The stability test was carried out by setting out samples under high humidity and light conditions. By detecting the content changes of lidocaine patch samples under various packaging materials, the most suitable packaging material for lidocaine patches was selected. The test results are shown in Table 3. :
[0034] Table 3 The impact of the type of lidocaine patch packaging material on the content of the preparation
[0035]
[0036]
[0037] The content of lidocaine in the lidocaine patch was 104.4% on the 0 day of stakeout. The results in Table 2 show that the content ...
Embodiment 3
[0044] The selection of embodiment 3 bupivacaine paste packaging materials
[0045] Since both bupivacaine and lidocaine can be used as local anesthetics, bupivacaine samples were used to carry out screening experiments on packaging materials. The stability test was carried out under the conditions of -30°C freezing, 40°C high temperature, 60°C high temperature, 92.5% high humidity and light conditions. By detecting the content changes of ropivacaine patch samples under various packaging materials, ropivacaine was selected The most suitable packaging material for patches, the test results are shown in Table 5
[0046] Table 5 The impact of the type of bupivacaine patch packaging material on the content of the preparation
[0047]
[0048]
[0049] The content of bupivacaine in the bupivacaine patch was 104.9% at day 0 of stakeout. The results in Table 5 show that the impact trend of the packaging material of the bupivacaine patch is similar to that of lidocaine and rop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com