CD38 antibody, chimeric antigen receptor and drug
A chimeric antigen receptor and antibody technology, which can be used in antibody mimics/scaffolds, drug combinations, anti-tumor drugs, etc., and can solve the problem of few types of CD38 antibodies.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
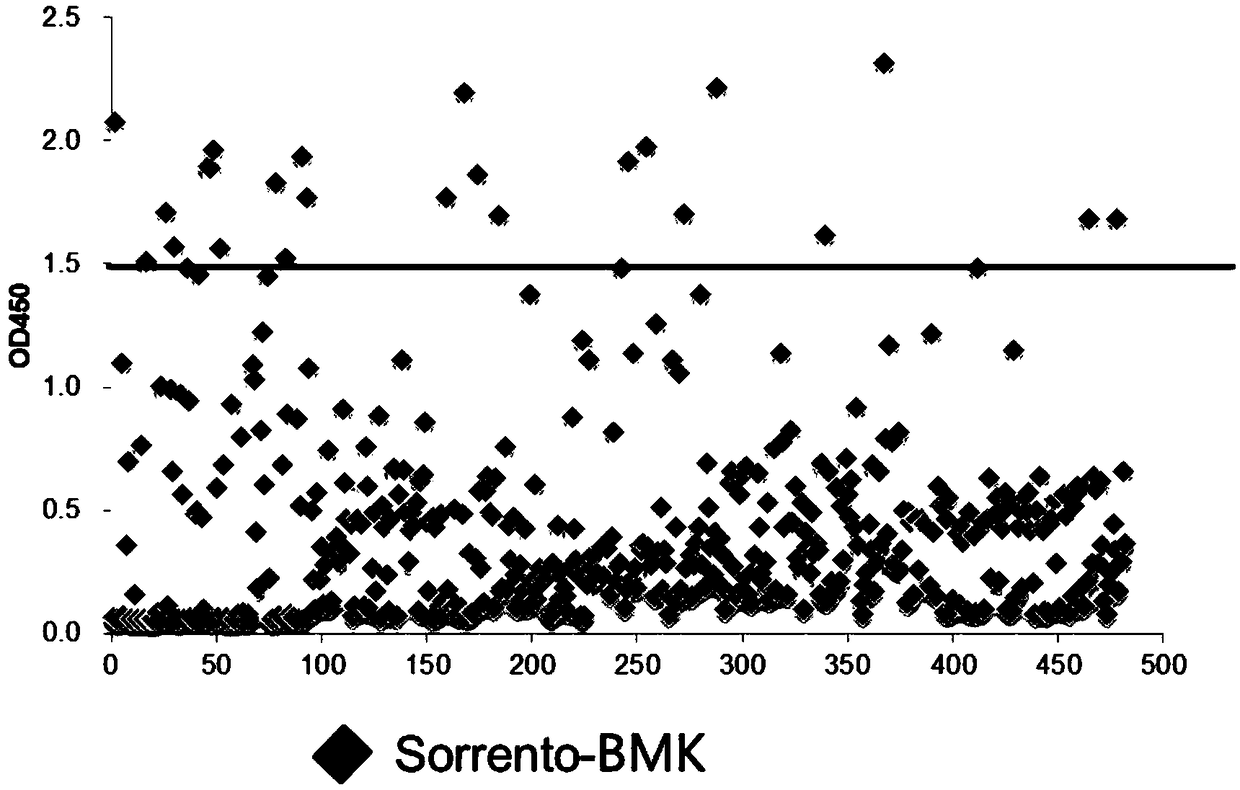
[0055] 1 Phage library panning
[0056] Biotinylated hCD38 protein was used as the panning antigen for the fully human antibody library. First, block the phage antibody at room temperature with blocking solution (PBST / 5% skimmed milk powder) for 2 h, and the input amount of phage is 2×10 12 Phage, then add 10μg antigen, incubate at room temperature for 1h, add 50μl pre-blocked M-280Streptavidin magnetic beads, incubated at room temperature for 30min.
[0057] First wash off the unbound phages with PBST, then elute the phages bound to the magnetic beads with 0.1M HCl-Glycine, then neutralize the eluate with Tris-HCl, and take part of the phages to infect the large intestine in the logarithmic growth phase Bacillus TG1, the collected phages were used for the next round of panning.
[0058] Gradually increase the screening intensity of each round, and stop panning when the enrichment degree reaches more than 100 times.
[0059] 2 Use phage Elisa to screen anti-CD38 single-ch...
Embodiment 2
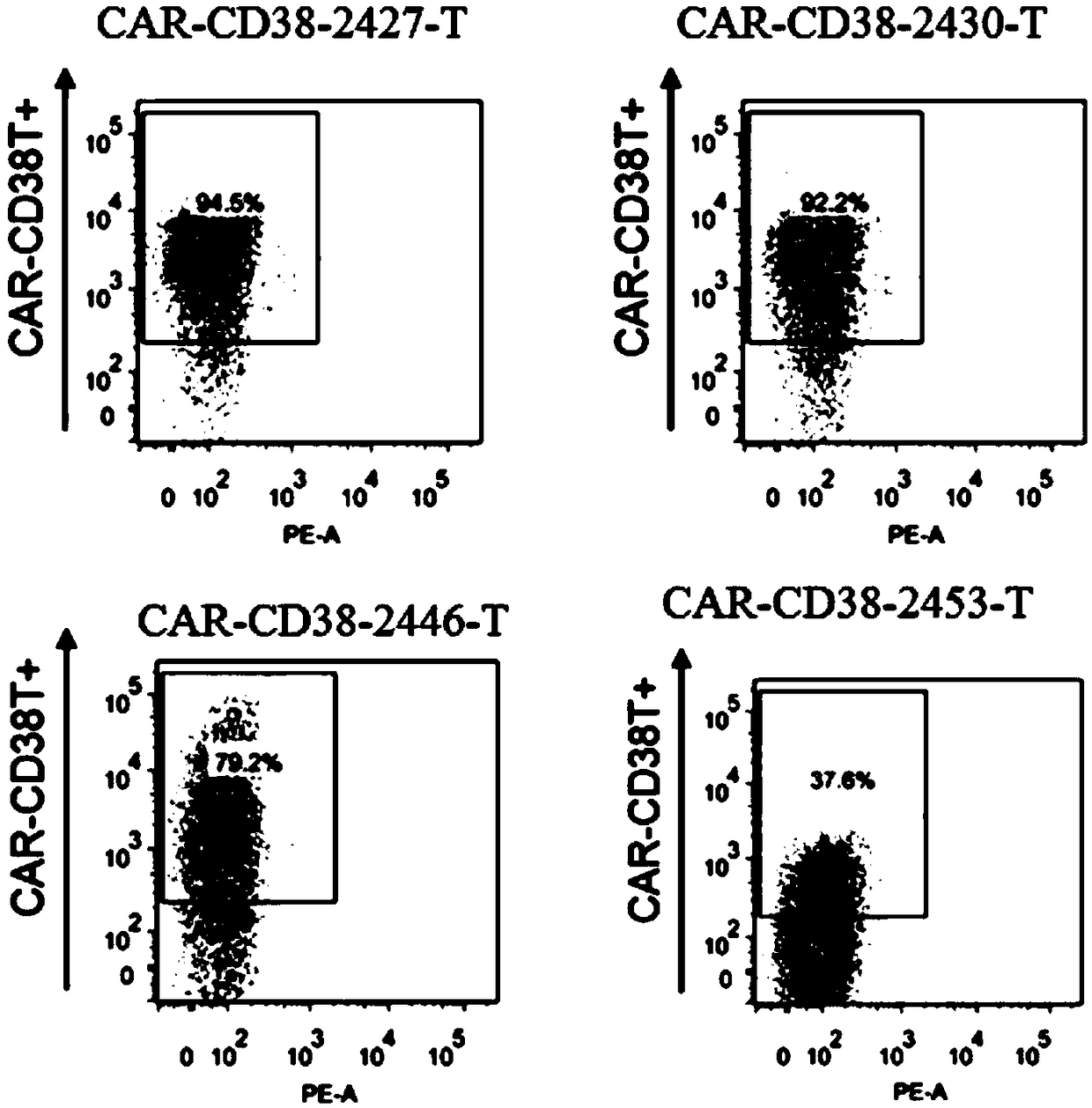
[0088] Construction of chimeric antigen receptor expression vector
[0089] Build method:
[0090](1) Whole gene synthesis: signal peptide (nucleotide sequence SEQ ID NO.66, amino acid sequence SEQ ID NO.73), CD38 antibody light chain variable region (light chain variable region of 2427, 2430, 2446 or 2453), Linker (nucleic acid sequence SEQ ID NO.67, amino acid sequence SEQ ID NO.74), CD38 antibody heavy chain variable region (heavy chain variable region of 2427, 2430, 2446 or 2453), hinge region (hinge) (nucleic acid sequence SEQ ID NO.74) ID NO.68, amino acid sequence SEQ ID NO.75), CD8α transmembrane domain (TM) (nucleic acid sequence SEQ ID NO.69, amino acid sequence SEQ ID NO.76), 4-1BB co-stimulatory signal transduction region (nucleic acid sequence SEQ ID NO.70, amino acid sequence SEQ ID NO.77) and CD3ζ signaling domain (nucleotide sequence SEQ ID NO.71, amino acid sequence SEQ ID NO.78).
[0091] The above-mentioned element sequences were connected sequentially to ...
Embodiment 3
[0099] Preparation of Strain Containing Chimeric Antigen Receptor Expression Vector
[0100] method:
[0101] (1) Take out DH5α competent cells from -80°C refrigerator and thaw on ice.
[0102] (2) Add 5ng of plasmid to the competent medium, mix gently, and place on ice for 5 minutes.
[0103] Plasmids are: pCDH-EF1-CAR-CD38-2427-copGFP, pCDH-EF1-CAR-CD38-2430-copGFP, pCDH-EF1-CAR-CD38-2446-copGFP, or pCDH-EF1-CAR-CD3-2453 -copGFP.
[0104] (3) Heat shock at 42°C for 90 seconds, and place on ice for 30 minutes.
[0105] (4) Add 0.5ml non-resistant LB, and incubate at 37°C, 180rpm for 30 minutes.
[0106] (5) Spread on an ampicillin-resistant plate.
[0107] (6) Invert overnight culture at 37°C.
[0108] (7) Pick a single clone and culture it in ampicillin-resistant LB at 37°C and 200 rpm for 9-12 hours.
[0109] (8) Glycerin was added to the bacterial solution, and the final concentration of glycerol was 10%, and the bacterial strain was stored in a refrigerator at -80°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com