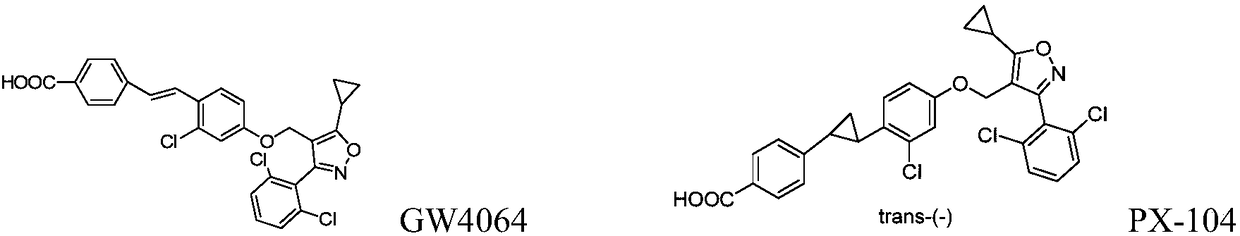
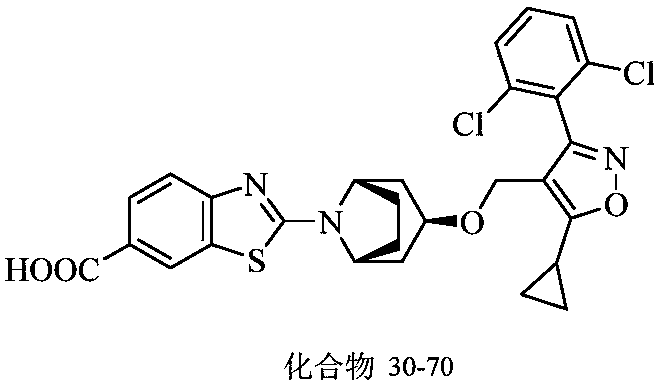
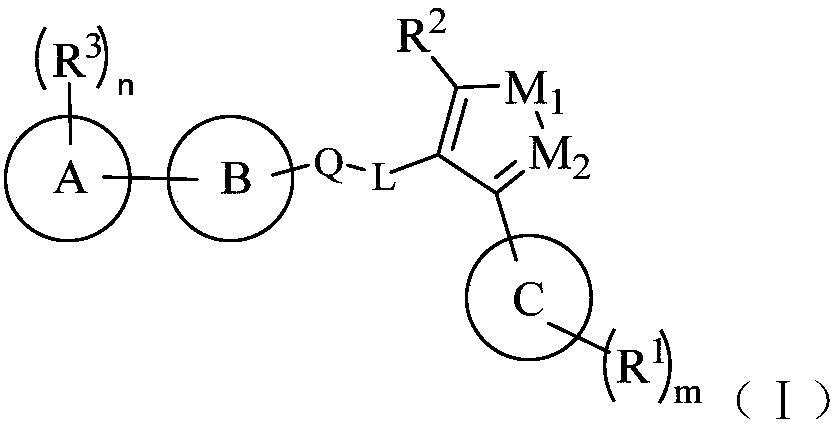
Farnesoid X receptor (FXR) stimulant
A stereoisomer, alkyl technology, applied in the field of FXR receptor agonists, can solve problems such as unknown structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0151] 1. Preparation of Intermediate 1
[0152] Dissolve starting material 1 (self-made or purchased) in an organic solvent (such as tetrahydrofuran, etc.), add starting material 2 (made or purchased), alkaline solution (such as potassium tert-butoxide, etc.) and 18-crown-6, React at 20°C-40°C. After the reaction was completed, the solvent was removed from the reaction liquid under reduced pressure, and purified by silica gel column chromatography to obtain intermediate 1.
[0153] 2. Preparation of Intermediate 2
[0154] Dissolve the intermediate 1 in an organic solvent (such as dichloromethane, etc.), add an acidic solution (such as trifluoroacetic acid, etc.), after the addition, stir the reaction at room temperature, after the reaction is complete, concentrate to obtain a crude product, or add the concentrated solution to the alkali neutral solution (such as saturated sodium bicarbonate solution, etc.), extracted with an organic solvent (such as ethyl acetate, etc.), t...
experiment example 1
[0170] Experimental Example 1: The effect of the compounds of the present invention on the relative expression of BSEP mRNA in HepG2 cells
[0171] Test substance: the compound of the present invention, its chemical name and preparation method are shown in the preparation examples of each compound.
[0172] Reagents: PBS: Phosphate buffer saline.
[0173] experimental method:
[0174] 1. Plating cells, adding compounds and collecting cells
[0175] Use trypsin to digest and collect the cells, and measure the cell concentration; according to the counting results, resuspend the cells to a density of 7.5e5cell / mL; inoculate 2 mL of cells in each well of a 6-well cell culture plate; place the culture plate in an incubator, at 37°C, 5%CO 2 Conditioned for 24 hours.
[0176] Dilute the compound to 3,0.3mM with DMSO; take 5ul of the stock solution diluted in the previous step and add it to 5ml of the culture medium. The concentrations of the obtained working solutions were 3 an...
experiment example 2
[0202] Experimental example 2: The metabolic stability experiment of liver microsomes of the compound of the present invention in different species
[0203] Test product: Compound 1 and Compound 3 of the present invention are self-made, and their chemical names and preparation methods are shown in the preparation examples of each compound.
[0204] Reference substance: compound 30-70, PX-104, prepared according to the method of the prior art, see the background art for its structure.
[0205] Experimental Materials:
[0206] The mixed liver microsomes of SD rats were purchased from XenoTech, the batch number is: 1410271, and the protein concentration of liver microsomal was 20 mg·mL -1 .
[0207] The mixed liver microsomes of Cyno monkeys were purchased from Reid Liver Disease Research Center (Shanghai Co., Ltd.), the batch number is: NMZC, and the concentration of liver microsomal protein is 20 mg·mL -1 .
[0208]Human mixed liver microsomes were purchased from XenoTech...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com