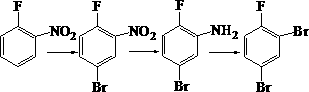
1,3-dibromo-4-fluorobenzene preparation method
A technology of fluorobenzene and o-fluoronitrobenzene is applied in the field of preparation of 1,3-dibromo-4-fluorobenzene, and can solve the problems of complicated subsequent separation, short process route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 2
[0018] (1) Bromination reaction: Add 1,200 g of o-fluoronitrobenzene and 6,000 mL of acetic acid as a solvent into the reaction vessel, control the temperature at about 15 degrees, and add 1,680 g of brominated reagent N-bromosuccinimide in batches. After the addition, stir for a period of time, GC tracking, until the reaction is complete. After the reaction was completed, it was analyzed with ice water and filtered with suction to obtain 1808 g of off-white solid 5-bromo-2-fluoronitrobenzene with a purity of 97% and a yield of 96.3%.
[0019] (2) Reduction reaction: Add 1420 g of iron powder and an appropriate amount of water into the reactor, stir, heat, control the temperature at about 90 degrees, and then add 1320 g of the nitro substance in the previous step in batches. After adding, GC traces until the reaction is complete. After the reaction was completed, cool, add ethyl acetate, filter with suction, separate the oil layer from the mother liquor, and rotary evaporate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com