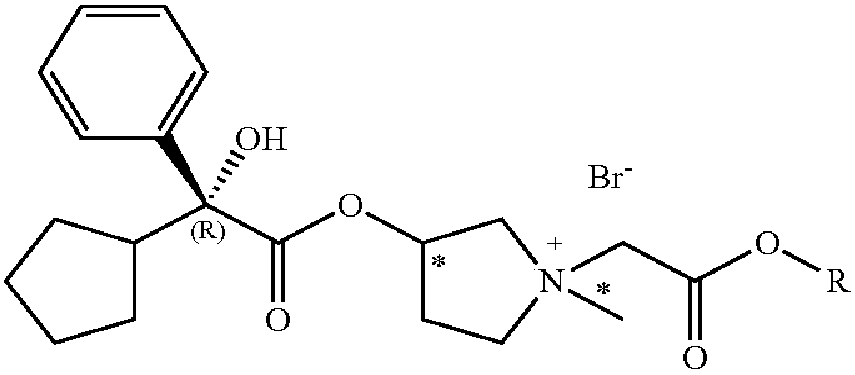
Anticholinergic glycopyrrolate esters for the treatment of hyperhidrosis
A topical application, phenyl technology, applied in the direction of ointment delivery, organic active ingredients, cosmetic preparations, etc., can solve the problems of repeated injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Experiments can be performed to demonstrate that the activity of the compounds of the invention in the treatment of hyperhidrosis is unexpectedly comparable to that of equivalent doses of glycopyrronium bromide.
[0095] Comparative sweat reduction in the armpit area
[0096] A 4% solution of soft glycopyrronium bromide (eg ethyl or methyl ester) in 70% ethanol was prepared (Solution 1).
[0097] A 4% solution of glycopyrronium bromide in 70% ethanol was prepared (Solution 2).
[0098] On Day 1, baseline sweat production assessments were determined during 4 consecutive 5 minute (min) periods each under sweat stimulation conditions (92°F, 60% humidity). Average Sweat Production will calculate and take into account the Baseline-5 Minute Sweat Volume.
[0099] The reduction in sweat production can be quantified as follows:
[0100] 0.5 ml of solution 1 was applied to the axillary area on day 1 at bedtime. On Day 2, approximately 8 hours after application of Solution 1...
Embodiment 2
[0104] Preparation of compound (v), i.e. (2R,3'R)3-(2-cyclopentyl-2-phenyl-2-hydroxyacetoxy)-1-(methoxycarbonylmethyl base)-1-methylpyrrolidine bromide 5% solution and tested its efficacy in reducing sweating in human subjects.
[0105] Axillary sweat production was measured gravimetrically: filter paper was weighed and then placed in the armpit for 5 minutes, then re-weighed to determine the amount of sweat produced (weight) during this time. The weight difference (dry weight) from the final weight of the filter paper was determined as the sweat production during this period.
[0106] Four independent assessments were performed for 5 minutes each (to reduce variability) and the mean was assessed.
[0107] The baseline is the mean of a total of 8 assessments (measured over 2 days, 4 assessments per day) for each 5 minute period without treatment with a compound of the invention.
[0108] Four once-daily doses of 0.5 ml of 5% glycopyrrolate compound solution were administer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com