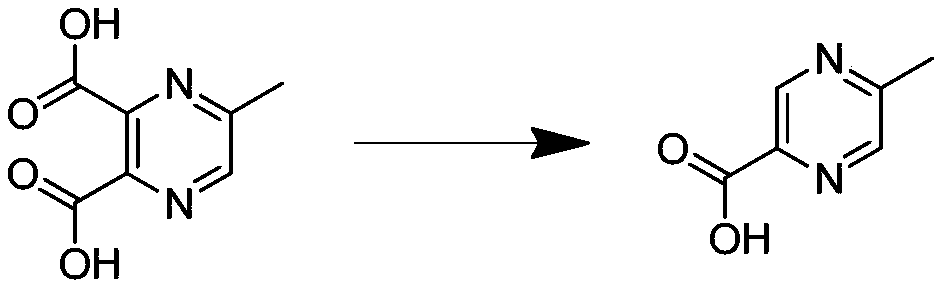
Synthetic process of 2-methyl-5-carboxylic acid pyrazine
A technology of methylpyrazine and pyrazine formate, applied in the field of medicine, can solve the problems of complex purification process, low yield of oxidation and decarboxylation, and poor selectivity of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of 3-Hydroxy-5-methylpyrazine-2-carboxamide
[0066] In a 500mL three-necked reaction flask, add 216g of 40% aqueous solution of aceglyoxal and 117g of 2-aminomalonamide in sequence, place in an ice-water bath, and cool down to about 5°C; while stirring, slowly add the prepared 100g of 40% sodium hydroxide aqueous solution, control the dropping rate, keep the internal temperature at 5°C, after 6 hours, add 10% hydrochloric acid solution dropwise to the system, adjust the pH value to about 6, a large amount of brownish yellow solid precipitates, filter, water After washing once, the filter cake was dried in an oven to obtain 120 g of crude product. LC purity 97%, yield 78%. 1 H-NMR (DMSO-d 6 ):δ=2.15(s,3H),7.41(s,1H),8.67(br,2H),15.62(br,1H).
[0067] Preparation of 3-Hydroxy-5-methylpyrazine-2-carboxylic acid
[0068] Add 120g of the crude product obtained in the previous step into a 500mL three-necked reaction flask, install a reflux condensing devi...
Embodiment 2
[0074] Preparation of 3-Hydroxy-5-methylpyrazine-2-carboxamide
[0075] In a 500mL three-necked reaction flask, add 216g of 40% aqueous solution of aceglyoxal and 117g of 2-aminomalonamide in sequence, place in an ice-water bath, and cool down to about 5°C; while stirring, slowly add 100g of the prepared solution dropwise to the system 40% sodium hydroxide aqueous solution, control the rate of addition, keep the internal temperature at 10°C, after 6 hours, add 10% hydrochloric acid solution dropwise to the system, adjust the pH value to about 6, precipitate a large amount of brownish yellow solid, filter and wash with water Once, the filter cake was dried in an oven to obtain 118g of crude product. LC purity 97%, yield 77%. 1 H-NMR (DMSO-d 6 ):δ=2.15(s,3H),7.41(s,1H),8.67(br,2H),15.62(br,1H).
[0076] Preparation of 3-Hydroxy-5-methylpyrazine-2-carboxylic acid
[0077] Add 119.23g of the crude product obtained in the previous step into a 500mL three-necked reaction fla...
Embodiment 3
[0083] Preparation of 3-bromo-5-methylpyrazine-2-carboxylic acid
[0084] 100g of 3-hydroxyl-5-methylpyrazine-2-carboxylic acid crude product prepared according to the method of Example 1 was added to a 500mL three-necked reaction flask, a reflux condenser was installed, 200mL xylene was added, 5mL DMF, Under stirring, add 129g of thionyl bromide dropwise from the constant pressure dropping funnel. After the addition, the system is heated up to 80°C for 6 hours of reaction; 200mL of water was added to the bottom solution, the mixture was extracted with 3*150mL butanone, the organic phases were combined and concentrated to obtain 93g of crude product. The crude product was recrystallized in 500 mL of water, and dried to obtain 85.2 g of a white solid product with a LC purity of 98% and a yield of 61%. 1 H-NMR (DMSO-d 6 ):δ=2.42(s,3H),8.86(s,1H),12.31(br,1H).
[0085] Preparation of 2-methyl-5-carboxylic acid pyrazine
[0086]Place 85g of the product obtained in the prev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com