Azaspirocyclic cation-loaded polybiphenyl basic membrane and preparation method thereof
A polybiphenyl alkaline and azaspiro-ring technology, which is applied in the field of preparation of alkaline anion exchange membranes for fuel cells, can solve the problems of chemical stability restricting life, and achieve excellent thermal and mechanical properties, low swelling, and high ion conductivity. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of 6-azaspiro[5.5]undecyl quaternary ammonium cation-supported polybiphenyl (PBP-ASU) basic membrane:
[0046] Its preparation process is as follows:
[0047]
[0048] (1) Preparation of polybiphenyl (PBP)
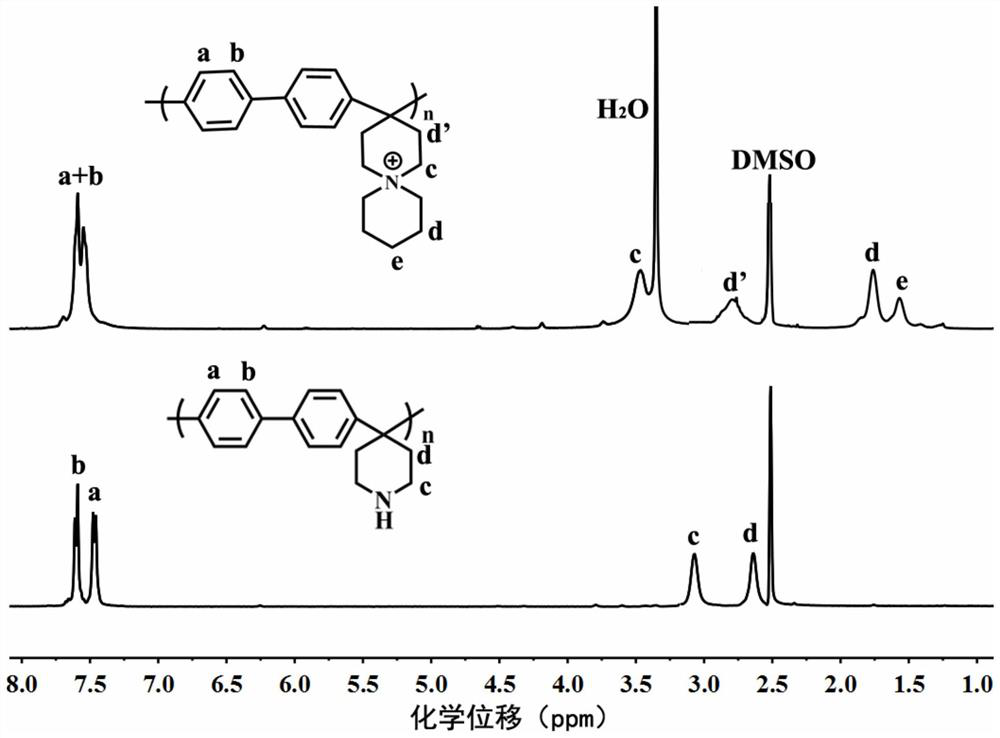
[0049] Take 3.0 grams of commercially available biphenyl and 3.2 grams of 4-piperidone, dissolve them in 10 milliliters of dichloromethane, add commercially available 3 milliliters of trifluoroacetic acid and 10 milliliters of trifluoromethanesulfonic acid, stir and react for 4 hours, complete reaction Finally, pour the viscous solution into the lye to precipitate, filter with suction to obtain a white solid, wash the polymer with deionized water several times to neutrality, finally, dry the polymer, collect the precipitate, and obtain a white polymer solid. Productive rate: 87%, degree of polymerization is 810, the nuclear magnetic spectrum of polybiphenyl (PBP) ( 1 HNMR) see figure 1 ;
[0050] (2) Take 3 grams of polybiphenyl (PBP) obtained in ...
Embodiment 2
[0057] Preparation of 6-azaspiro[5.5]undecyl quaternary ammonium cation-supported polyterphenyl (PTP-ASU) basic membrane:
[0058] Its preparation process is as follows:
[0059]
[0060] (1) Preparation of polyterphenyl (PTP)
[0061] Take 3.0 grams of commercially available terphenyls and 2.2 grams of 4-piperidone, dissolve them in 10 milliliters of dichloromethane, add 2 milliliters of commercially available trifluoroacetic acid and 7 milliliters of trifluoromethanesulfonic acid, stir and react for 6 hours, after complete reaction , pour the viscous solution into lye to precipitate, filter with suction to obtain a white solid, wash the polymer with deionized water several times until neutral, finally, dry the polymer, collect the precipitate, and obtain a white polymer solid poly Terphenyl (PTP), polymerization degree is 1400, productive rate: 91%;
[0062] (2) Get 3 grams of polyterphenyl (PTP) obtained in step (1), dissolve it in acetone, and add 0.7 grams of commerc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com