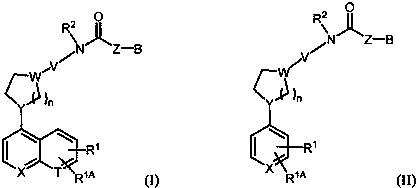
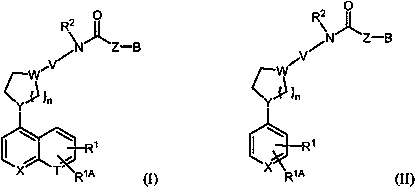
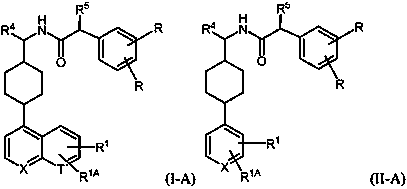
Inhibitors of indoleamine 2,3-dioxygenase and methods of their use
A -OH, C1-C6 technology, applied in the field of enzymatically active compounds, can solve problems such as different functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0063] In another embodiment, the present invention provides a combination comprising one or more compounds of the present invention and / or pharmaceutically acceptable salts thereof, stereoisomers thereof, tautomers thereof or solvates thereof thing.
[0064] In another embodiment, the present invention provides a compound comprising a pharmaceutically acceptable carrier and at least one compound of the present invention and / or a pharmaceutically acceptable salt thereof, a stereoisomer thereof, a tautomer thereof or Pharmaceutical compositions of its solvates.
[0065] In another embodiment, the present invention provides a pharmaceutical composition comprising a pharmaceutically acceptable carrier and a therapeutically effective amount of at least one compound of the present invention and / or pharmaceutically acceptable salts, stereoisomers thereof body, its tautomer or its solvate.
[0066] In another embodiment, the present invention provides a process for the preparation ...
Embodiment 1
[0208] N-((1-(6-fluoroquinolin-4-yl)piperidin-4-yl)methyl)-2-( right- Tolyl)acetamide
[0209]
[0210] 1A. Tert-butyl ((1-(6-fluoroquinolin-4-yl)piperidin-4-yl)methyl)carbamate
[0211] To a homogeneous mixture of 4-chloro-6-fluoroquinoline (220.0 mg, 1.2 mmol) in anhydrous NMP (4 mL) in a sealable vial was added (piperidin-4-ylmethyl)carbamate tert-Butyl ester (350.0 mg, 1.6 mmol) followed by DIPEA (0.8 mL, 4.6 mmol). The vial was sealed and the mixture was stirred at 60°C for 2 hours, then at 90°C for 17 hours, then at 120°C for 24 hours. After cooling to room temperature, the reaction mixture was purified by Isco chromatography to afford tert-butyl ((1-(6-fluoroquinolin-4-yl)piperidin-4-yl)methyl)carbamate as an off-white solid ( 323.7 mg; 74% yield). MS (ES): m / z = 360 [M+H] + . t R = 0.71 min ( Method B ). 1 H NMR (400MHz, DMSO-d 6 ) δ8.66 (d, J =5.0 Hz, 1H), 8.01 (dd, J =9.1, 5.7 Hz, 1H), 7.66 - 7.51 (m, 2H),7.01 (d, J =4.9 Hz, 1H), 6.93 (t, J =5.7 H...
Embodiment 2
[0217] 2-(4-fluorophenyl)-N-((1-(6-fluoroquinolin-4-yl)piperidin-4-yl)methyl)acetamide
[0218]
[0219] Under nitrogen atmosphere, (1-(6-fluoroquinolin-4-yl)piperidin-4-yl)methanamine TFA salt (1B, 41.8 mg, 0.09mmol) in anhydrous THF (1 mL) and To a heterogeneous mixture in dioxane (0.5 mL) was added DIPEA (0.06 mL, 0.3 mmol) followed by 2-(4-fluorophenyl)acetyl chloride (15.5 mg, 0.09 mmol). After stirring at ambient temperature for 15 hours, the mixture was diluted with DMSO, filtered through a syringe filter, and purified by preparative HPLC / MS to afford the title compound (6.1 mg; 18% yield). MS (ES): m / z = 396 [M+H] + . t R = 1.19 min ( Method A ). 1 H NMR (500MHz, DMSO-d 6 ) δ 8.60 (d, J =6.7 Hz, 1H), 8.23 - 8.19 (m, 1H), 8.02 (dd, J =9.2, 5.1 Hz, 1H), 7.90 - 7.84 (m, 1H), 7.76 (d, J =9.9 Hz, 1H), 7.31 - 7.26(m, 2H), 7.15 (d, J =6.7 Hz, 1H), 7.09 (t, J =8.8 Hz, 2H), 4.03 (d, J =12.4 Hz,2H), 3.41 (s, 2H), 3.31 (t, J =12.2 Hz, 2H), 3.10 - 3.00 (m, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com