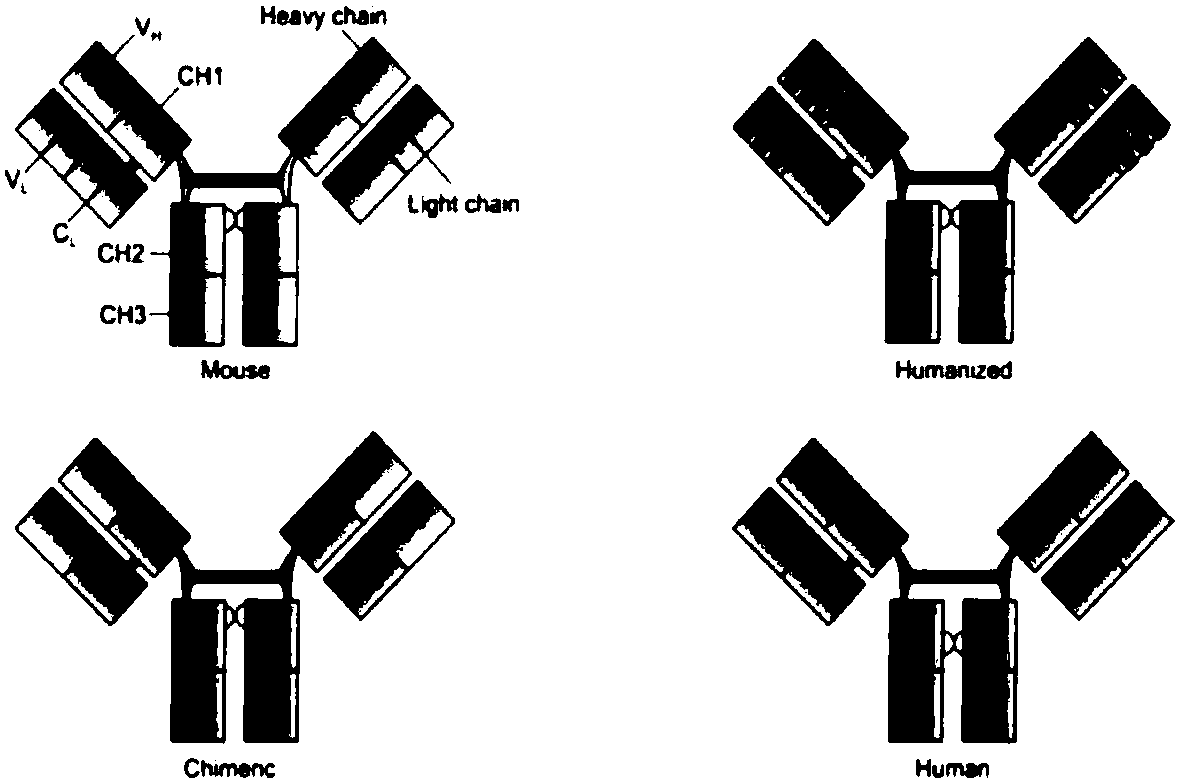
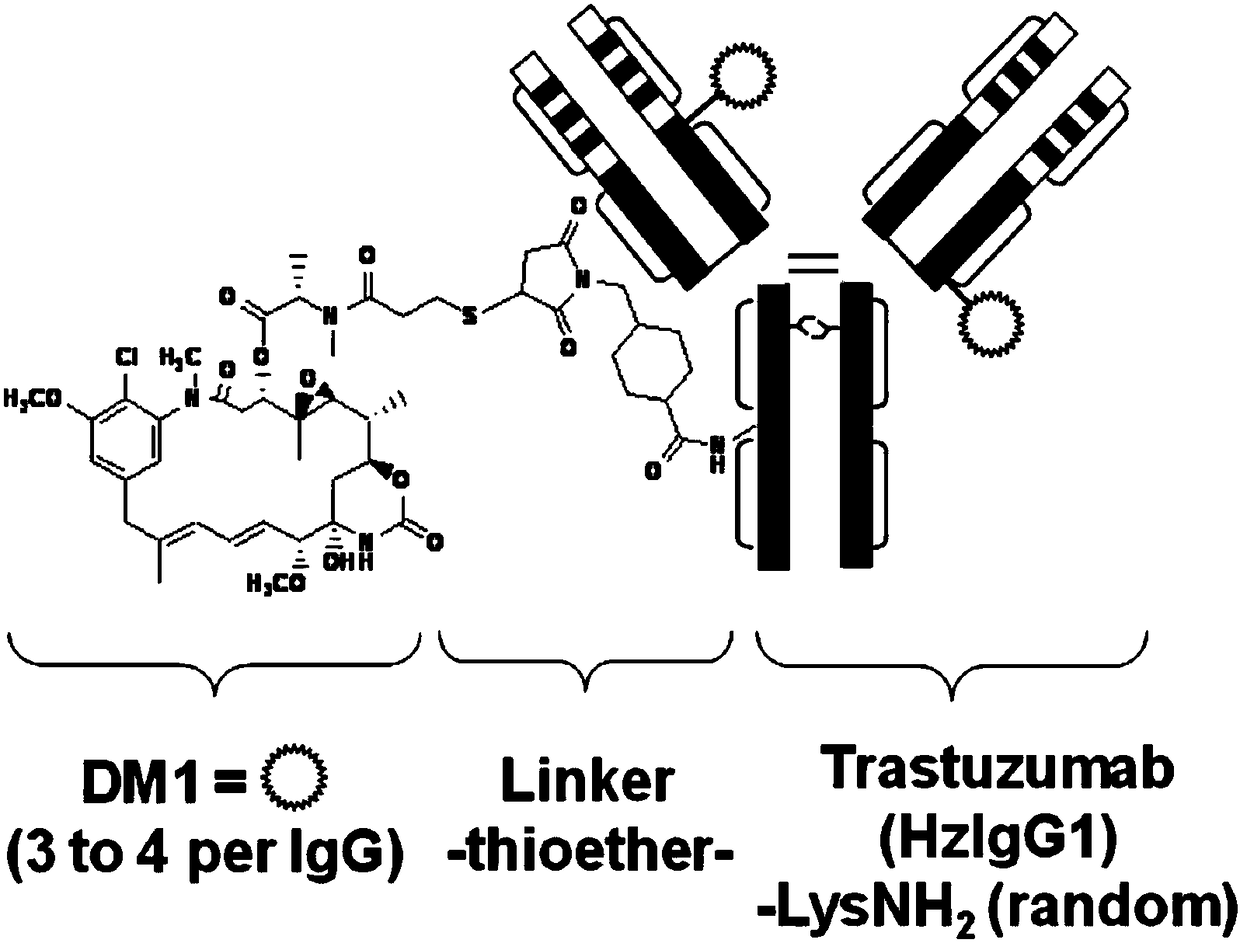
Development and application of fully human or humanized bombesin receptor GRPR monoclonal antibody drug or diagnostic reagents
A technology for monoclonal antibodies and diagnostic reagents, which is applied in the fields of medicine and clinical testing, and can solve the problems that the development of fully human or humanized monoclonal antibodies has not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[0066] Camptothecin-carbonyl-Pro-DSer-Nle-DTyr-DSer-mAb
[0067] Camptothecin-carbonyl-Hydroxyproline-mAb
[0068] Camptothecin-thioether-Lys-DSer-DSer-DSer-DSer-DSer-Pro-DSer-Nle-DTyr-DSer-mAb
[0069] Methotrexate-CH2CO-DLys-DTyr-Lys-mAb
[0070] Methotrexate-thioether-mAb
[0071] Thiocolchicine-Thioether-DSer-Nle-DTyr-DSer-mAb
[0072] Thiocolchicine-Carbonyl-Sar-DSer-Nle-DTyr-DSer-mAb
[0073] Oligo(DNA)-S-S-mAb
[0074] More GRPR monoclonal antibodies (mAb) are connected to various bioactive molecules (A) through linkers (linkers) (B) to form more antibody complexes, for example (see below):
[0075] The biologically active molecule A (A-B-mAb) linked to the fully human or humanized GRPR monoclonal antibody can be, but not limited to, any known therapeutic agents or cytotoxic agents. For example, antineoplastic agents such as: Acivicin; Aclarubicin; AcodazoleHydrochloride; Acronine; Adozelesin; Adriamycin; Aldesleukin; Anthramycin; Asparaginase; Asperlin; Azacitidi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com