A method for determining the loading amount of continuous flow chromatography and its application
A definite method, flow chromatography technology, applied in the direction of peptide preparation methods, chemical instruments and methods, instruments, etc., can solve the problems such as the inability to determine the sample volume, achieve faster data processing speed, greater convenience, and take into account the yield and the effect of filler loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
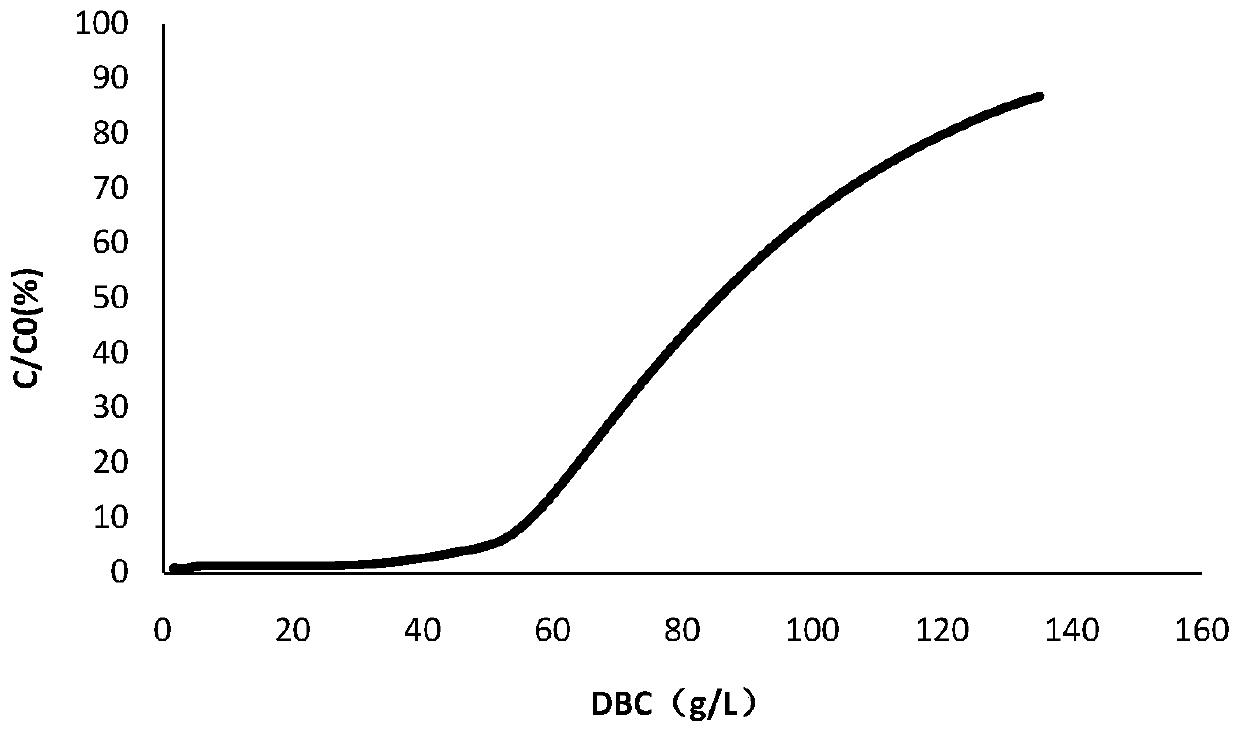
[0051] This example provides a method for determining the loading amount of monoclonal antibodies expressed by CHO cells separated and purified by continuous flow chromatography. In the crude monoclonal antibody expressed by CHO cells to be purified, the expression amount is 3 g / L The pH of the sample was 7.0, and the conductivity was 15 mS / cm.
[0052] The method for determining the loading amount of the present embodiment includes the following steps:
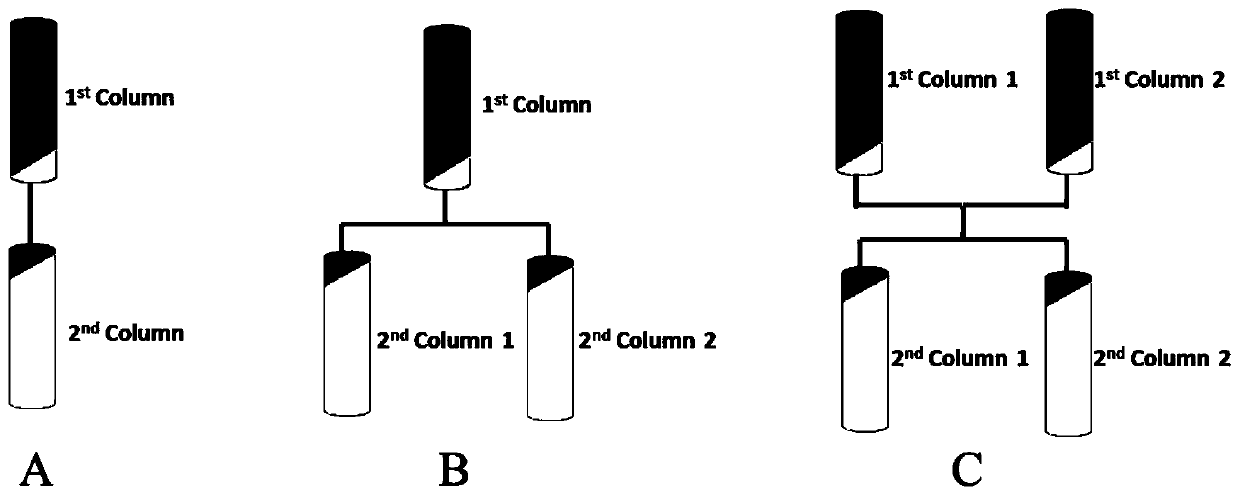
[0053] (1) Take 1 g of monoclonal antibody protein expressed by CHO cells purified by single-column chromatography, adjust the pH to 7.0, conductance 15 mS / cm, and use equilibrium solution (50 mM NaAc-HAc, 150 mM NaCl, pH 7.4 for protein concentration) ) was diluted to 3g / L, and two columns were applied in series; wherein, the filler of the chromatography column was Mabselect Sure LX of GE, the column volume was 5mL (1.1cm*5.3cm), and the retention time was 2min;
[0054] (2) adopt the equilibration solution of 5CV (50mM NaA...
Embodiment 2
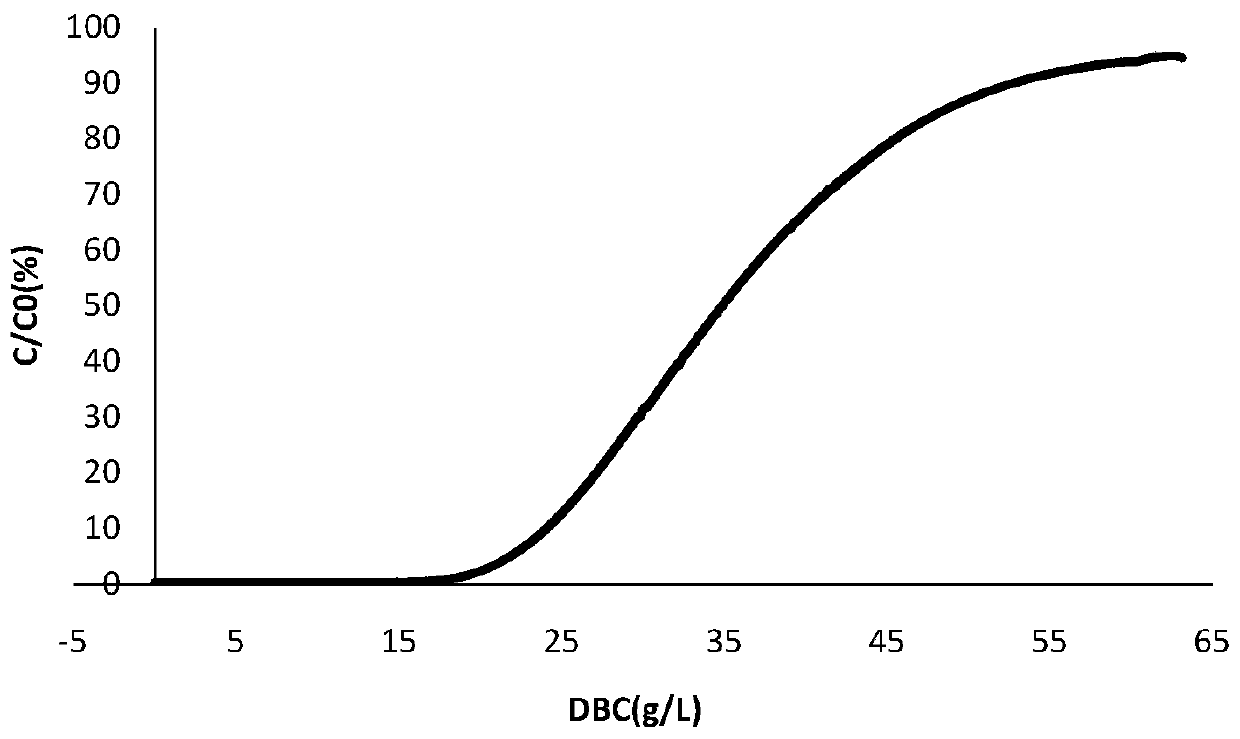
[0062] This example provides a method for determining the loading amount of monoclonal antibodies expressed by CHO cells separated and purified by continuous flow chromatography. In the crude monoclonal antibody expressed by CHO cells to be purified, the expression amount is 3.3 g / L , the loading pH is 7.2, and the conductivity is 15 mS / cm.
[0063] The method for determining the loading amount of the present embodiment includes the following steps:
[0064] (1) Take 1 g of monoclonal antibody protein expressed by CHO cells purified by single-column chromatography, adjust the pH to 7.2, conductance 15 mS / cm, and use equilibrium solution (50 mM NaAc-HAc, 150 mM NaCl, pH 7.4 for protein concentration) ) was diluted to 3.3g / L, and one column was used to drag two columns in series for sample loading; wherein, the chromatography column was HiTrap MabSelect SuRe 1mL prepacked column of GE Company, and the retention time was 2min;
[0065] (2) adopt the equilibration solution of 5CV (...
Embodiment 3
[0073] This example provides a method for determining the loading amount of monoclonal antibodies expressed by CHO cells separated and purified by continuous flow chromatography. In the crude monoclonal antibody expressed by CHO cells to be purified, the expression amount is 0.5 g / L , the loading pH was 7.15, and the conductance was 14 mS / cm.
[0074] The method for determining the loading amount of the present embodiment includes the following steps:
[0075] (1) Take 1 g of monoclonal antibody protein expressed by CHO cells purified by single-column chromatography, adjust the pH to 7.15, conductance 14 mS / cm, and use equilibrium solution (50 mM NaAc-HAc, 150 mM NaCl, pH 7.4 for protein concentration) ) was diluted to 0.5g / L, and two columns were dragged in series for loading; wherein, the packing of the chromatography column was Eshmuno A of Merck Company, the column volume was 5mL (1.1cm*5.3cm), and the retention time was 2min;
[0076] (2) adopt the equilibration solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com