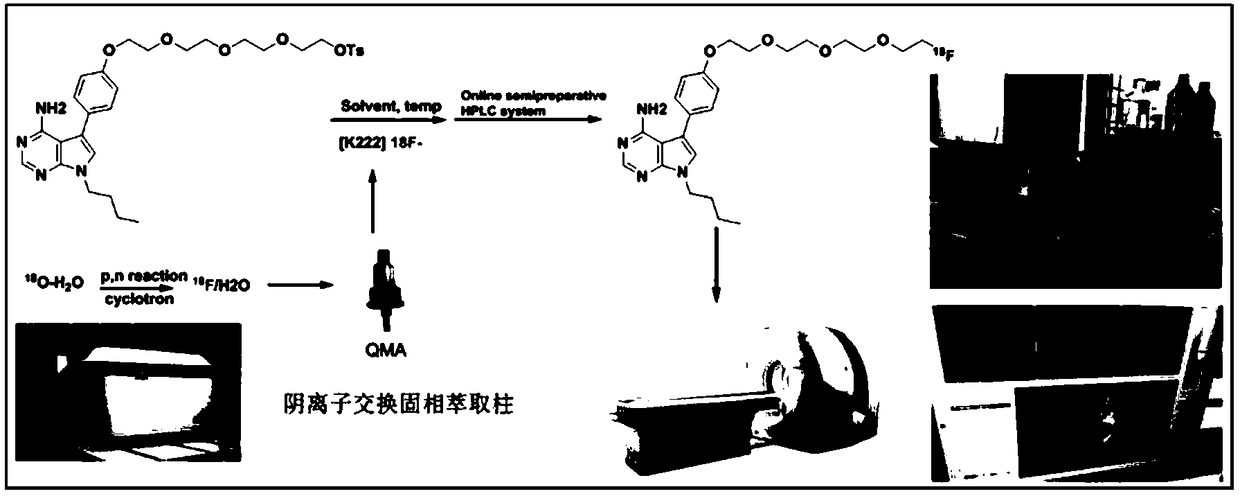
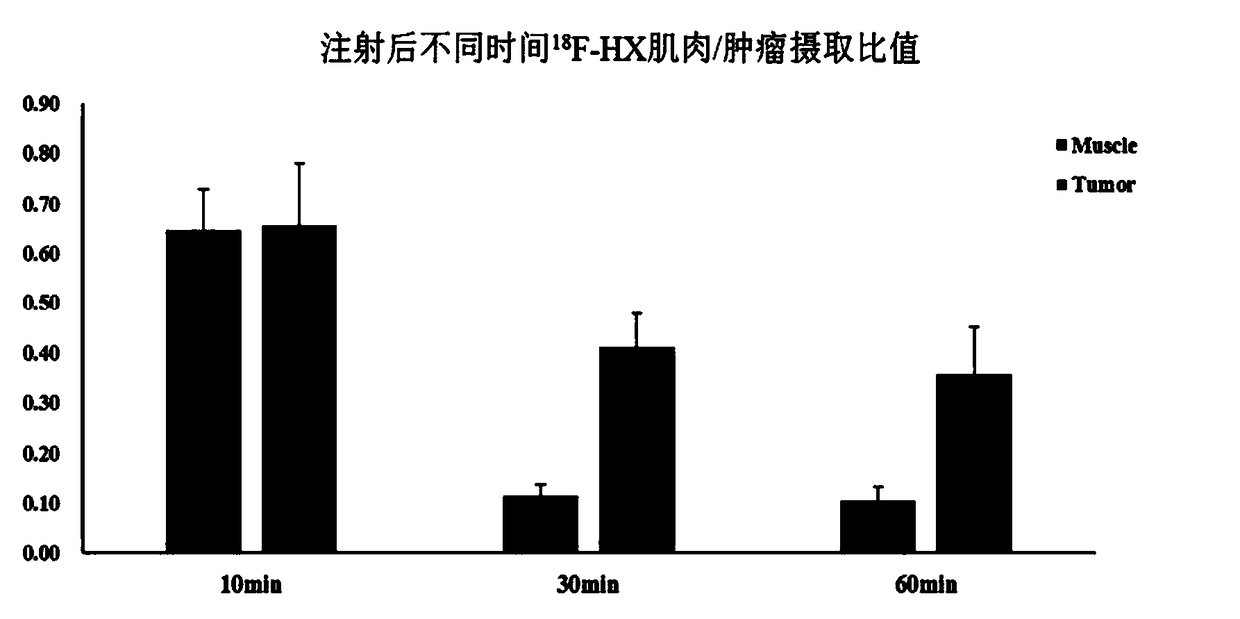
18F-PET/CT tracer with 7-deazaneplanocin base as parent nucleus, and preparation method thereof
A technology of 18F-HX and tracer, applied in organic chemical methods, chemical instruments and methods, preparations for in vivo tests, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 compound 1
[0057]
[0058] (1) Synthesis of 5-iodo-7-n-butyl-7H-pyrrolo[2,3-d]pyrimidine 4-chloro (compound 4):
[0059] 4-Chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine (compound 3) (5g, 17.90mmol) was mixed with cesium carbonate (11.66g, 4.66mmol) and dissolved in 30mL DMF and placed in ice water , after the mixture was cooled to 0°C, iodo-n-butane (6.17 mL, 53.7 mmol) was added. After 10 min, the mixture was taken out from the ice water and placed at room temperature and stirred for 5 h, and the reaction progress was detected by TLC. After the reaction was completed, the mixture was cooled and 60 mL of ice water was added, and precipitation occurred. After filtration and drying, 5.52 g of a yellow solid (compound 4) was obtained (91.9% yield).
[0060] Compound 4: 1 H NMR (600MHz, DMSO-d6) δ8.64(s, 1H), 8.06(s, 1H), 4.27(t, J=7.1Hz, 2H), 1.82–1.74(m, 2H), 1.22(h, J=7.4Hz, 2H), 0.88(t, J=7.4Hz, 3H). 13 C NMR(151MHz,DMSO-d6)δ162.75,1...
Embodiment 218
[0067] Example 2 18 Preparation of F-HX compounds
[0068]
[0069] 1. Preparation of Compound 2
[0070] Tetraethylene glycol bis(p-toluene hydrochloride) (164mg, 3.46mmol), compound 1 (100mg, 3.46mmol) and anhydrous potassium carbonate (100mg, 3.46mol) were heated under reflux in 50mL benzene solution for 4h, and in 1N HCl After and, the reaction mixture was extracted with dichloromethane. The solution was then dried under reduced pressure to obtain a solid, which was separated by column chromatography to obtain 160 mg of a brown-yellow syrupy product (compound 2) (yield 76%).
[0071] Compound 2: 1 H NMR (600MHz, Chloroform-d)δ8.29(s,1H),δ7.84-6.97(m,8H,arom-H),6.95(s,1H),5.52(s,2H),4.23-4.21 (t,J=7.2Hz,2H),4.23-3.59(t,J=7.2Hz,16H),δ2.44(s,3H),1.87-1.80(m,2H),1.44-1.35(m,2H ),0.97-0.95(t,J=7.4Hz,3H).HR-MS(ESI+): Calc.for[C 31 h 40 N 4 o 7 S]: 613.2618[M+H]+; Found 613.2694[M+H]+, 635.2521[M+Na]+.
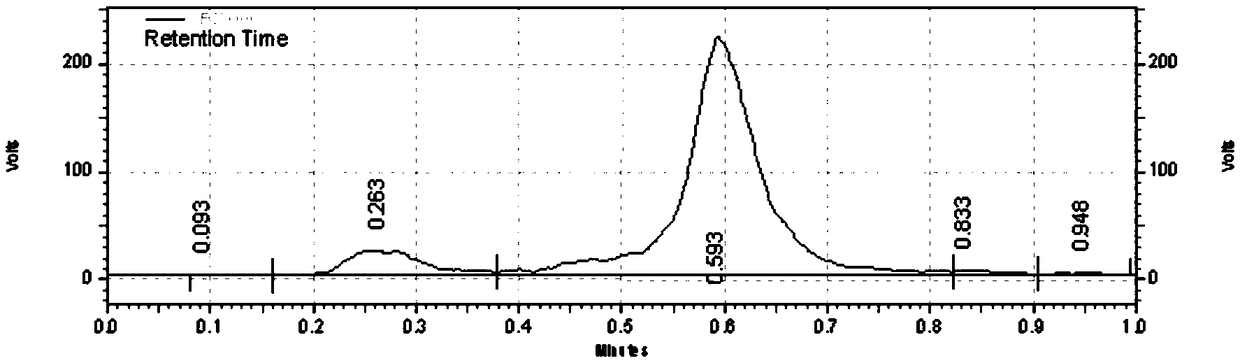
[0072] 2, 18 Preparation of F-HX
[0073] The radionuclide la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com