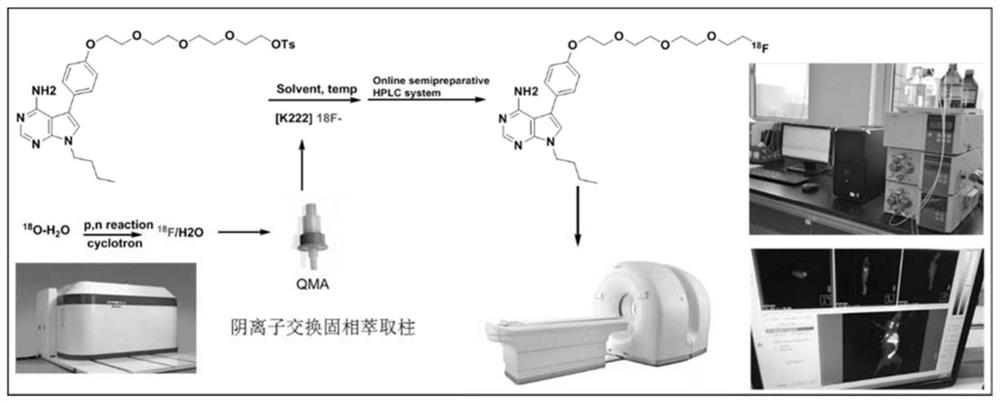
A kind of 18f-pet/ct tracer with 7-deaza adenine base as mother nucleus and preparation method thereof
A technology of 18F-HX and tracer, applied in organic chemical methods, chemical instruments and methods, preparations for in vivo tests, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation of Compound 1
[0057]
[0058] (1) Synthesis of 5-iodine-7-n-butyl-7H-pirarrol and [2,3-D] pyrimidine 4-chlorine (Compound 4):
[0059] 4-chloro-5-iodo-7H-pyrrole [2,3-D] pyrimidine (Compound 3) (5 g, 17.90 mmol) is mixed with carbonate (11.66 g, 4.66 mmol) mixed in 30 ml DMF and placed in ice water The mixture was cooled to 0 ° C, and iodine n-butane (6.17 mL, 53.7 mmol) was added. After 10 min, the mixture was taken out from ice water to the room temperature seal stirring for 5 h, and the reaction process was detected with TLC. After the reaction is completed, the mixture is cooled and 60 ml of ice water is added, and a precipitate occurs. Abovement, yellow solid (Compound 4) was dried (Compound 4) 5.52 g (yield was 91.9%).
[0060] Compound 4: 1 H NMR (600 MHz, DMSO-D6) Δ8.64 (S, 1H), 8.06 (S, 1H), 4.27 (T, J = 7.1 Hz, 2H), 1.82-1.74 (m, 2H), 1.22 (H, J = 7.4 Hz, 2H), 0.88 (t, j = 7.4 Hz, 3H). 13 C NMR (151MHz, DMSO-D6) δ162.75, 151.34, 150.87, 150...
Embodiment 218
[0067] Example 2 18 Preparation of F-HX compound
[0068]
[0069] 1. Preparation of Compound 2
[0070] Tetrate bis (toluene hydrochloride) (164 mg, 3.46 mmol), compound 1 (100 mg, 3.46 mmol) and anhydrous potassium carbonate (100 mg, 3.46 mol) were heated in a 50 mL benzene solution to heat 4 h, 1N HCl Afterward, the reaction mixture was extracted with dichloromethane. The solution was then dried under reduced pressure to give a solid after solid column chromatography (Compound 2) 160 mg (yield of 76%).
[0071] Compound 2: 1 H NMR (600MHz, Chloroform-D) δ8.29 (S, 1H), δ 7.84-6.97 (M, 8H, Arom-H), 6.95 (S, 1H), 5.52 (S, 2H), 4.23-4.21 (t, j = 7.2Hz, 2H), 4.23-3.59 (t, j = 7.2 Hz, 16H), δ2.44 (S, 3H), 1.87-1.80 (m, 2H), 1.44-1.35 (m, 2H ), 0.97-0.95 (T, J = 7.4 Hz, 3H) .hr-ms (ESI +): Calc.for [c 31 Hide 40 N 4 O 7 S]: 613.2618 [M + H] +; Found 613.2694 [M + H] +, 635.2521 [M + NA] +.
[0072] 2, 18 Preparation of F-HX
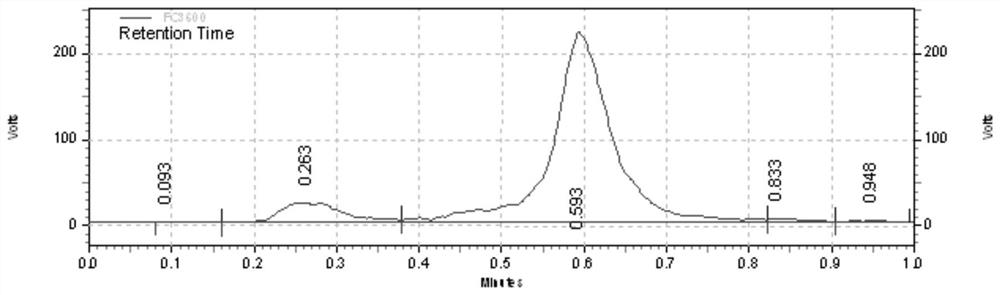
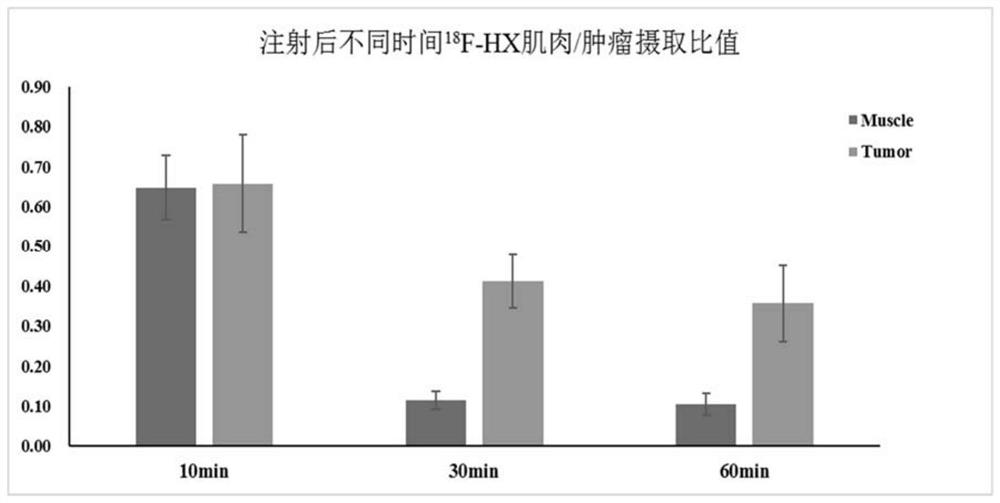
[0073] Radionic nuclei marking of Compound 1 figure 1 Ind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com