Unsaturated fluorocarbon, preparation method and application thereof
A technology of fluorocarbons and compounds, which is applied in the field of unsaturated fluorocarbons and its preparation, can solve problems such as low glass transition temperature, poor multilayering ability, and obstacles to 5G communication terminals, and achieve excellent dielectric properties, excellent Effect of mechanical and dielectric properties, good thermal and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
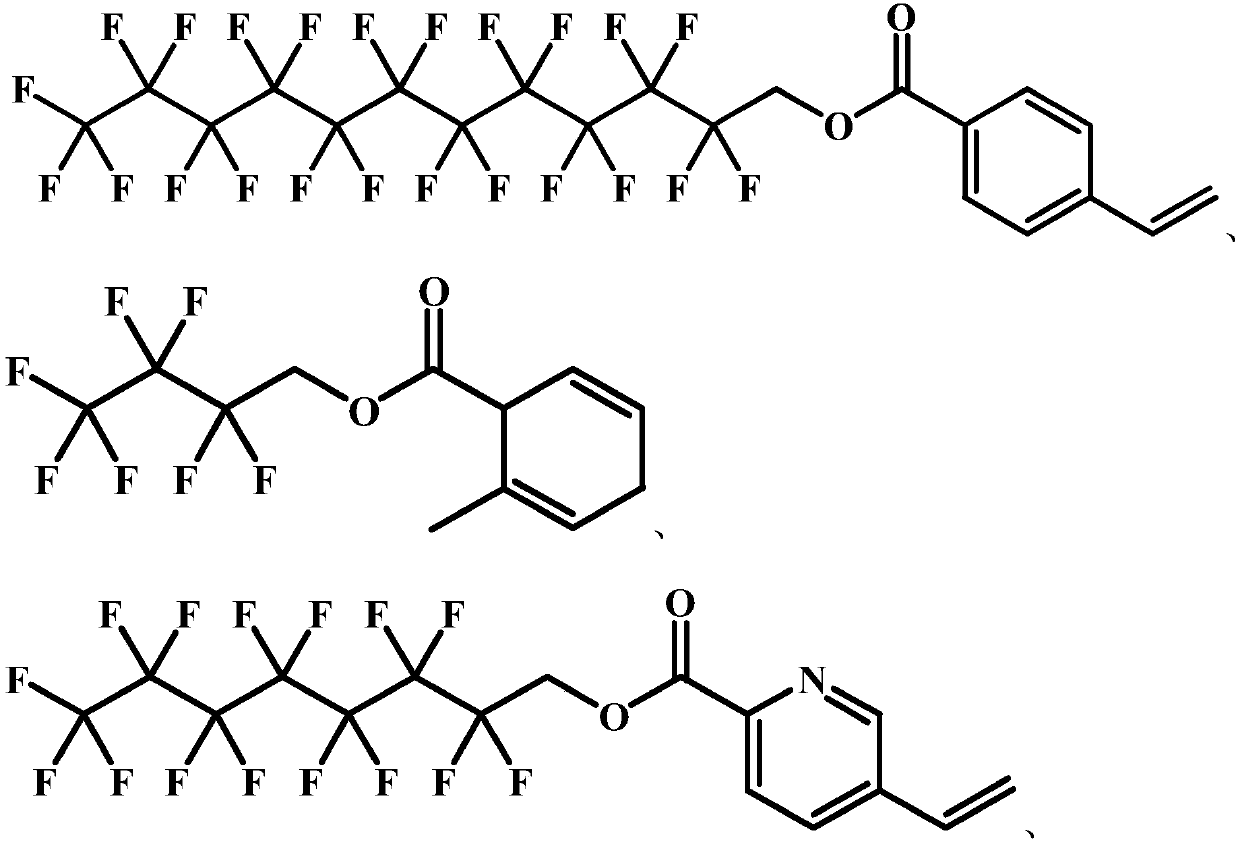
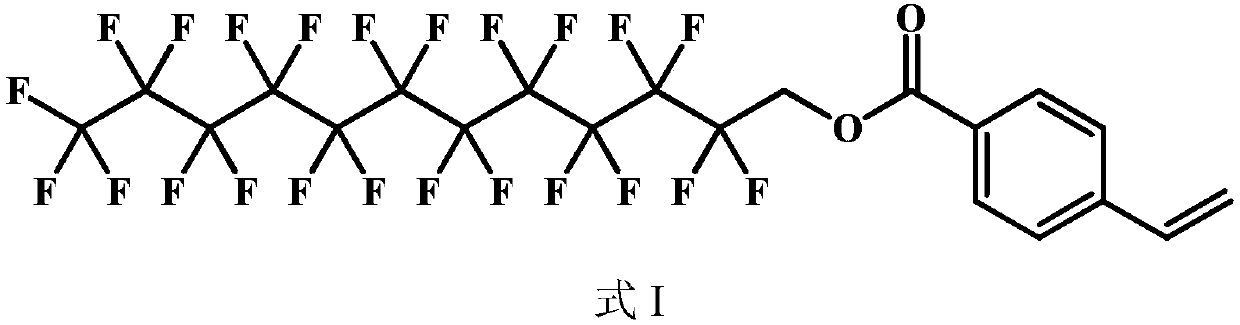
[0040] The present invention provides an unsaturated fluorocarbon compound whose structure is as shown in formula I:
[0041]
[0042] The preparation method of the compound represented by formula I is as follows: mix 1 mol of perfluorododecanol with 1.2 mol of 4-vinyl-benzoic acid, add 100 mL of 0.1 mol / L sodium hydroxide solution, and heat the reaction at 80°C for 2 hours with mechanical stirring, and cool To room temperature, wash with 500 mL of water 5 times, separate liquids, add anhydrous sodium sulfate, stand for 2 hours, and filter to obtain the compound represented by formula I.
[0043] 1 H NMR(CDCl 3 ,500MHz): δ7.95~7.89(m, 2H, Ar-H), 7.43~7.36(m, 2H, Ar-H), 6.65~6.60(m, H, CH 2 =C H ), 5.63~5.57(t, H, C H 2 =CH), 5.21~5.16(t, H, C H 2 =CH), 4.62~4.55(s, 2H, CH 2 ).
Embodiment 2
[0045] The present invention provides an unsaturated fluorocarbon compound whose structure is as shown in formula II:
[0046]
[0047] The preparation method of the compound represented by formula II: mix 1 mol perfluorobutanol with 1.2 mol 2-methyl-1,4-dihydrobenzoic acid, add 100 mL of 0.1 mol / L sodium hydroxide solution, and mechanically stir at 80°C The reaction was heated for 2.5 hours, cooled to room temperature, washed with 500 mL of water 5 times, separated, added with anhydrous sodium sulfate, allowed to stand for 2 hours, and filtered to obtain the compound represented by formula II.
[0048] 1 H NMR(CDCl 3 ,500MHz): δ5.58~5.48(m, H, CH=CH), 5.38~5.30(t, H, C H =C), 4.45~4.39(s, 2H, CH 2 ), 3.71~3.65(d,H,CH), 2.67~2.60(t,2H,CH 2 ), 1.75~1.68(t, 3H, CH 3 ).
Embodiment 3
[0050] The present invention provides an unsaturated fluorocarbon compound whose structure is as shown in formula III:
[0051]
[0052] The preparation method of the compound represented by formula III is: mixing 1 mol of perfluorooctanol with 1.2 mol of 5-vinyl-2-picolinic acid, adding 100 mL of 0.1 mol / L sodium hydroxide solution, and heating and reacting at 70°C with mechanical stirring for 3 hours, Cooled to room temperature, washed with 500 mL of water 5 times, separated liquids, added anhydrous sodium sulfate, allowed to stand for 2 hours, and filtered to obtain the compound represented by formula III.
[0053] 1 H NMR(CDCl 3 ,500MHz): δ9.03~8.96(m, H, Ar-H), 8.23~8.15(m, 2H, Ar-H), 6.66~6.61(m, H, CH 2 =C H ), 5.71~5.65(t, H, C H 2 =CH), 5.28~5.22(t, H, C H 2 =CH), 4.67~4.61(s, 2H, CH 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Critical surface tension | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com