Preparation method for 2-cyano-5-bromopyridine
A technology of bromopyridine and dibromopyridine, applied in the field of preparation of 2-cyano-5 bromopyridine, can solve problems such as many reaction steps, cumbersome steps, unfavorable industrialized production, etc., and achieve the effect of few steps and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
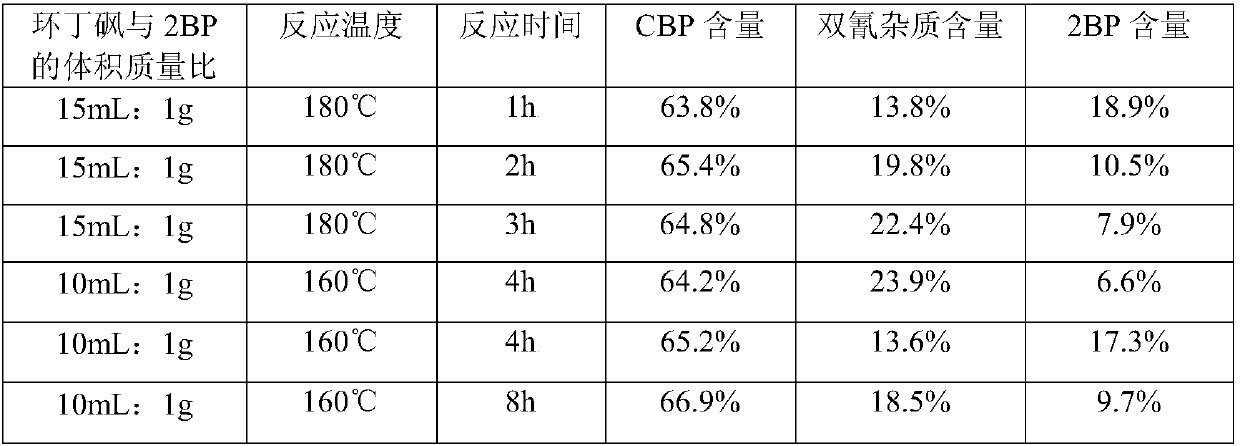
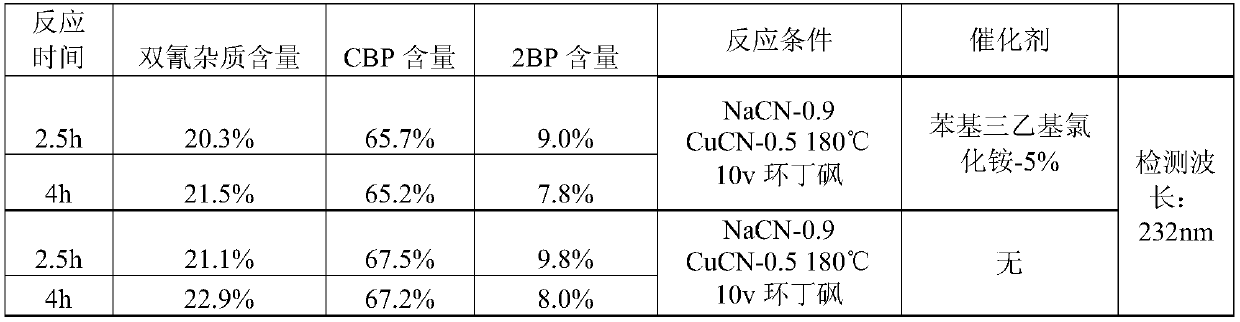
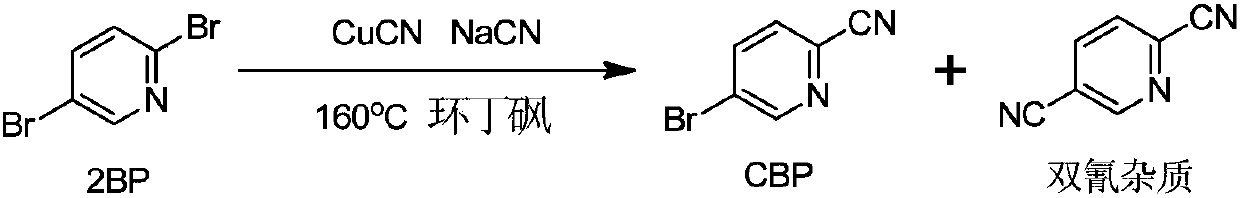
[0082] (1) Preparation of 2-cyano-5-bromopyridine (CBP) crude product
[0083]
[0084] 100g 2BP (0.422mol), 18.61g sodium cyanide (0.380mol) and 1261g (1000mL) sulfolane were sequentially added to a 2L four-necked flask. When the temperature was raised to 160° C., 11.34 g of cuprous cyanide (0.127 mol) was added, and the reaction was incubated for 6 hours, and the reaction endpoint was monitored by HPLC. After the reaction was complete, the oil pump was distilled at 160-170°C under reduced pressure, and about 1350 g of a colorless or slightly yellow fraction was collected. Quickly add 2500g of purified water dropwise to the distillate, the solvent sulfolane is miscible with water, and a white solid is precipitated. After the drop is completed, cool down to 0-5°C and keep stirring for 1h. After filtration, the filter cake was air-dried at 60° C. for 6-8 hours to obtain 54 g of a white crude product with a yield of 70%.
[0085] HPLC (240nm) detection: CBP: 93.6%; dicyanid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com