Long-acting coagulation factor vii and methods of producing same
A technology of active factors and molecules, applied in coagulation/fibrinolytic factors, chemical instruments and methods, prolonging plasma life fusion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0440] FVII-CTP 3 Feasibility study in FVIII-deficient hemophiliac mice
[0441] Tested FVII-CTP, FVII-CTP 2 and FVII-CTP 3 Study of harvest PK profile and coagulation activity relative to commercial FVII. Relative to FVII-CTP and FVII-CTP 2 Harvest or rhFVII, FVII-CTP 3 Exhibits an improved PK profile while maintaining its coagulation activity. To further characterize FVII-CTP 3 characterized in vitro and in vivo, generated small stable libraries of expressed and secreted proteins, and developed purification and activation procedures.
[0442] In the current study, FVIIa-CTP was tested in FVIII-deficient mice 3 pharmacokinetic and pharmacodynamic properties. Evaluate the PK profile of the protein. Establish a PK profile based on FVIIa-specific activity and compare with commercial products Compare. In addition, FVIIa-CTP after tail null transection (survival study) was tested 3 Long-lasting in vivo hemostatic ability to induce coagulation in FVIII-deficient mice.
...
Embodiment 2
[0530] Biochemical Properties of MOD-5014 Relative to Commercial Recombinant hFVIIa - Effect of Carboxy-Terminal Peptide (CTP) on Factor VIIa Activity
[0531] Project Rationale and Summary
[0532] These studies were designed to evaluate the biochemical properties of MOD-5014 relative to commercial recombinant hFVIIa (referred to herein as MOD-5000).
[0533] Research checks:
[0534] Cleavage of synthetic substrate of MOD-5014
[0535] Tissue factor (TF) binding of MOD-5014 measured by synthetic substrate cleavage
[0536] TF binding of MOD-5014 measured by Factor X (FX) activation
[0537] Kinetics of FX activation by TF-bound MOD-5014
[0538] Lipid binding of MOD-5014 measured by FX activation
[0539] Kinetics of factor activation by lipid-bound MOD-5014
[0540] Inactivation of MOD-5014 by antithrombin (AT)
[0541] Inactivation of MOD-5014 by TFPI
[0542] The overall data suggest that MOD-5014 has a similar mechanism of action with slightly reduced catalytic a...
Embodiment 3
[0625] Production of CTP-modified activator VII
[0626] Purpose
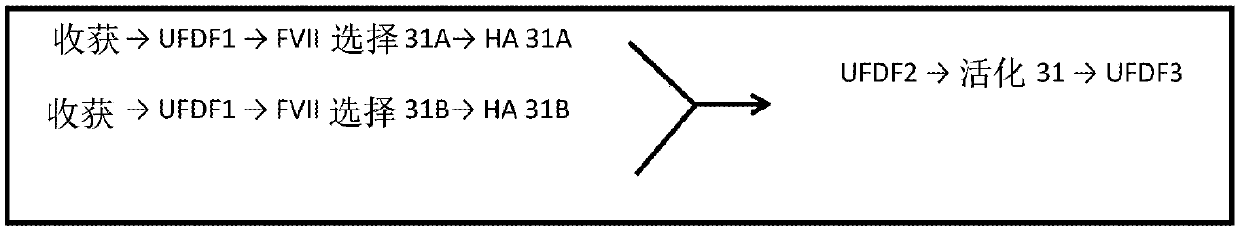
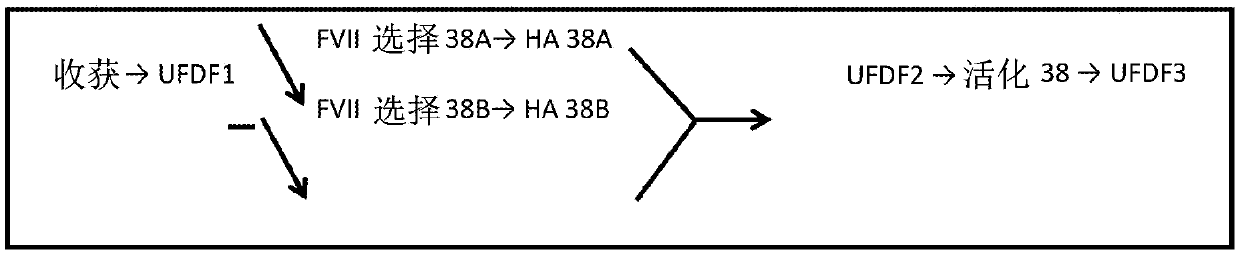
[0627] The purpose of the preparative method was to develop a fed-batch upstream method using CHO cells in a chemically defined medium by recombinant DNA technology followed by a robust and scalable downstream method for the purification of hyperglycosylated and hyper-γ-carboxylated MOD-5014. In other words, produce and purify MOD-5014 with the highest γ-carboxylation content and effectively remove process and production related impurities. It is important to analyze O-glycans, N-glycans, percent sialic acid, oxidation-associated forms, potency (tested by STA-CLOT assay), percent Gla domain (or percent uncarboxylated glutamic acid residues , and the percentage of unactivated FVII).
[0628] production process
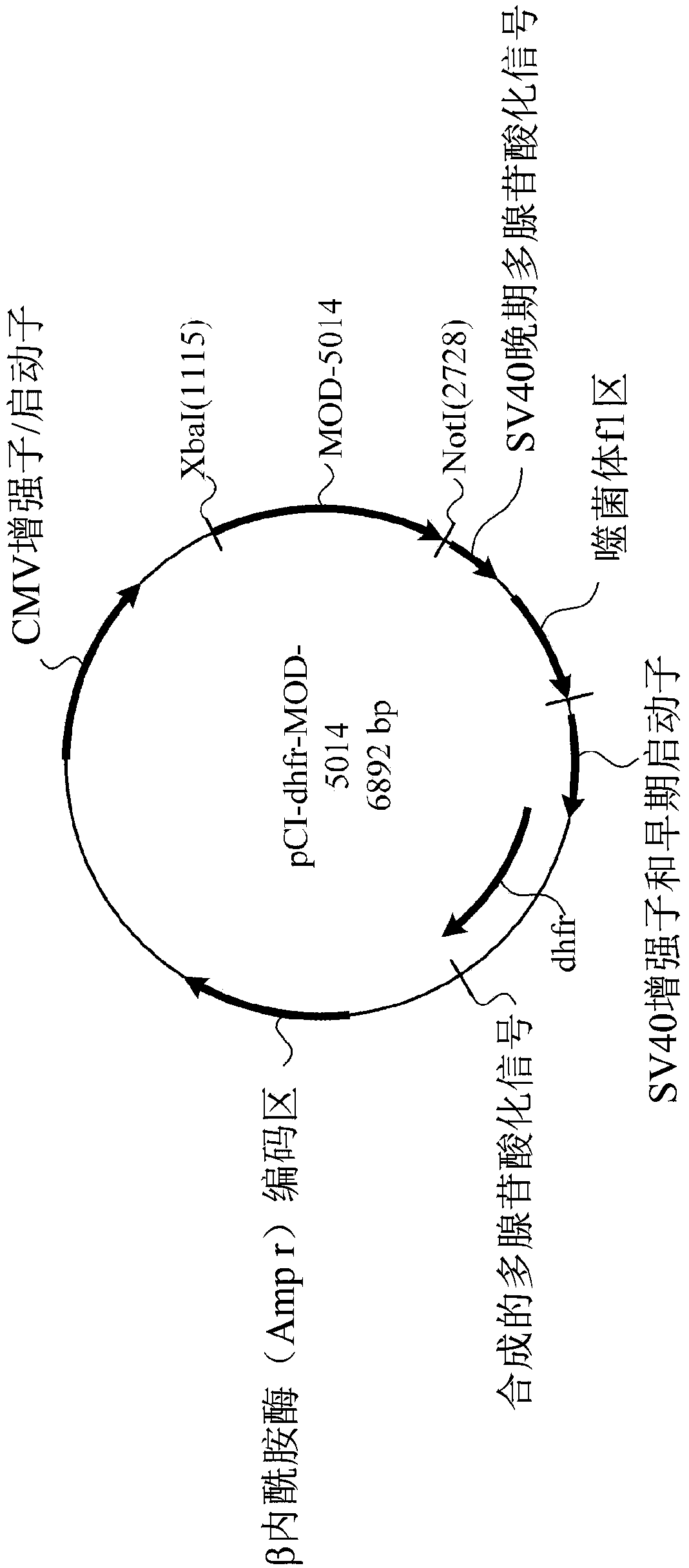
[0629] Transfection and stable clone selection
[0630] The cDNA encoding MOD-5014 was transfected into CHO cells (dhfr-negative CD DG44 cells suitable for growth in protein-free media and suspension ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com