Liquid drug injection containing ropivacaine mesylate and preparation method thereof
A technology of ropivacaine mesylate and water injection, which is applied in the field of drug preparation, can solve the problems of easy change, high cost, and dextro-isomer production, and achieve convenient transportation, good stability, and isomer little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
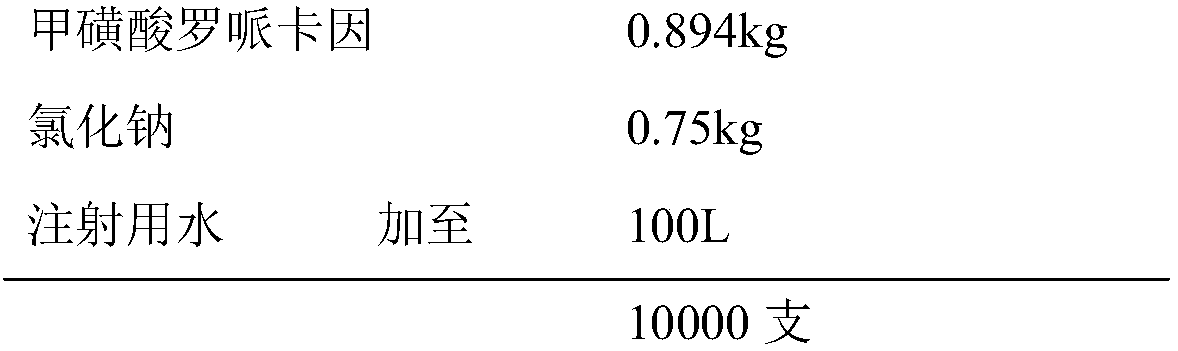
[0015] prescription:
[0016]
[0017] Preparation:
[0018] (1) Add 80L of water for injection in the dispensing tank, cool down to 40°C, add 0.75kg of sodium chloride and 0.894kg of ropivacaine mesylate, and stir for 30 minutes; the ropivacaine mesylate It was stored at 0°C after being recrystallized with methanol at 5°C before being formulated into an aqueous injection. After visually observing that the raw materials are completely dissolved, add 0.03 kg of wetted activated carbon for needles, and stir and adsorb for 20 minutes. (2) Use 0.01mol / L sodium hydroxide solution to adjust the pH value to 5.5, add water for injection to the full amount, and pull the loop for 20 minutes. (3) Sampling and testing the pH value, content and endotoxin of semi-finished products. (4) After the semi-finished product is qualified, the three-in-one equipment is used to make bottles and fill them with N 2 Filling, sealing. (5) Sterilize at 121°C for 15 minutes, cool down to below 45°C...
Embodiment 2
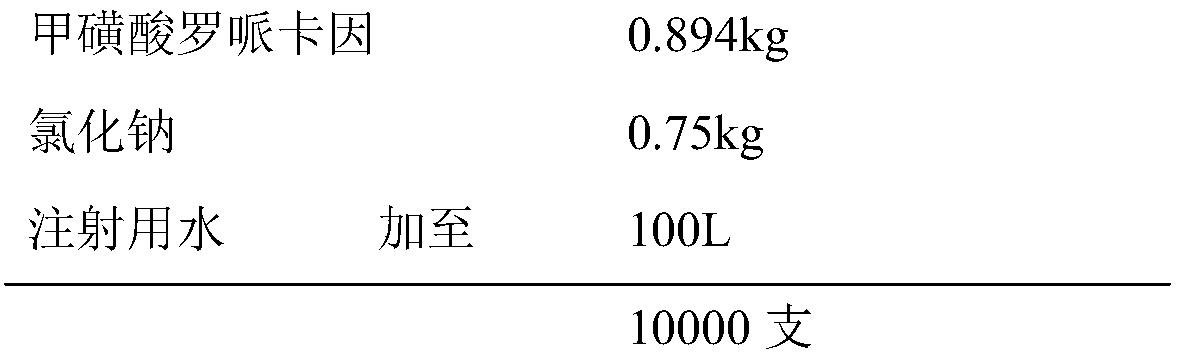
[0020] prescription:
[0021]
[0022] Preparation:
[0023] (1) Add 80L of water for injection in the dispensing tank, cool down to 40°C, add 0.75kg of sodium chloride and 0.894kg of ropivacaine mesylate, and stir for 30 minutes; the ropivacaine mesylate It was stored at -40°C after being recrystallized with methanol at 5°C before being formulated into an aqueous injection. After visually observing that the raw materials are completely dissolved, add 0.03 kg of wetted activated carbon for needles, and stir and adsorb for 20 minutes. (2) Use 0.01mol / L sodium hydroxide solution to adjust the pH value to 5.5, add water for injection to the full amount, and pull the loop for 20 minutes. (3) Sampling and testing the pH value, content and endotoxin of semi-finished products. (4) After the semi-finished product is qualified, the three-in-one equipment is used to make bottles and fill them with N 2 Filling, sealing. (5) Sterilize at 121°C for 15 minutes, cool down to below 45...
Embodiment 3
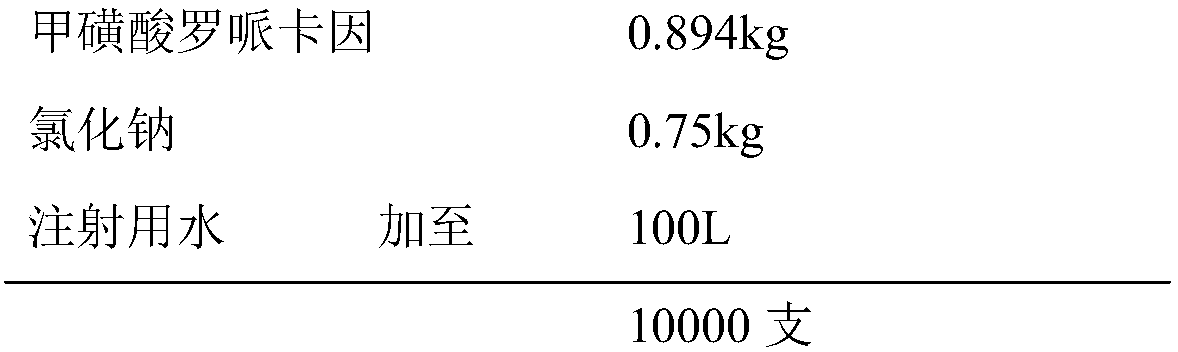
[0025] prescription:
[0026]
[0027] Preparation:
[0028] (1) Add 80L of water for injection in the dispensing tank, cool down to 40°C, add 0.75kg of sodium chloride and 0.894kg of ropivacaine mesylate, and stir for 30 minutes; the ropivacaine mesylate It was stored at -80°C after being recrystallized with methanol at 5°C before being formulated into an aqueous injection. After visually observing that the raw materials are completely dissolved, add 0.03 kg of wetted activated carbon for needles, and stir and adsorb for 20 minutes. (2) Use 0.01mol / L sodium hydroxide solution to adjust the pH value to 5.5, add water for injection to the full amount, and pull the loop for 20 minutes. (3) Sampling and testing the pH value, content and endotoxin of semi-finished products. (4) After the semi-finished product is qualified, the three-in-one equipment is used to make bottles and fill them with N 2 Filling, sealing. (5) Sterilize at 121°C for 15 minutes, cool down to below 45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com