Rabies vaccine virus screening method and preparation method of rabies vaccine
A technology of rabies vaccine and screening method, which is applied in the field of preparation of rabies vaccine and screening of rabies vaccine virus, can solve the problems of long adaptation period, etc., and achieve the effect of increasing yield and shortening adaptation period of subculture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The invention discloses a preparation method of a rabies virus vaccine, and those skilled in the art can refer to the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0041]The disclosed embodiments are described to enable any person skilled in the art to make or use the invention. Various modifications to these embodiments will be readily apparent to those ...
Embodiment 1
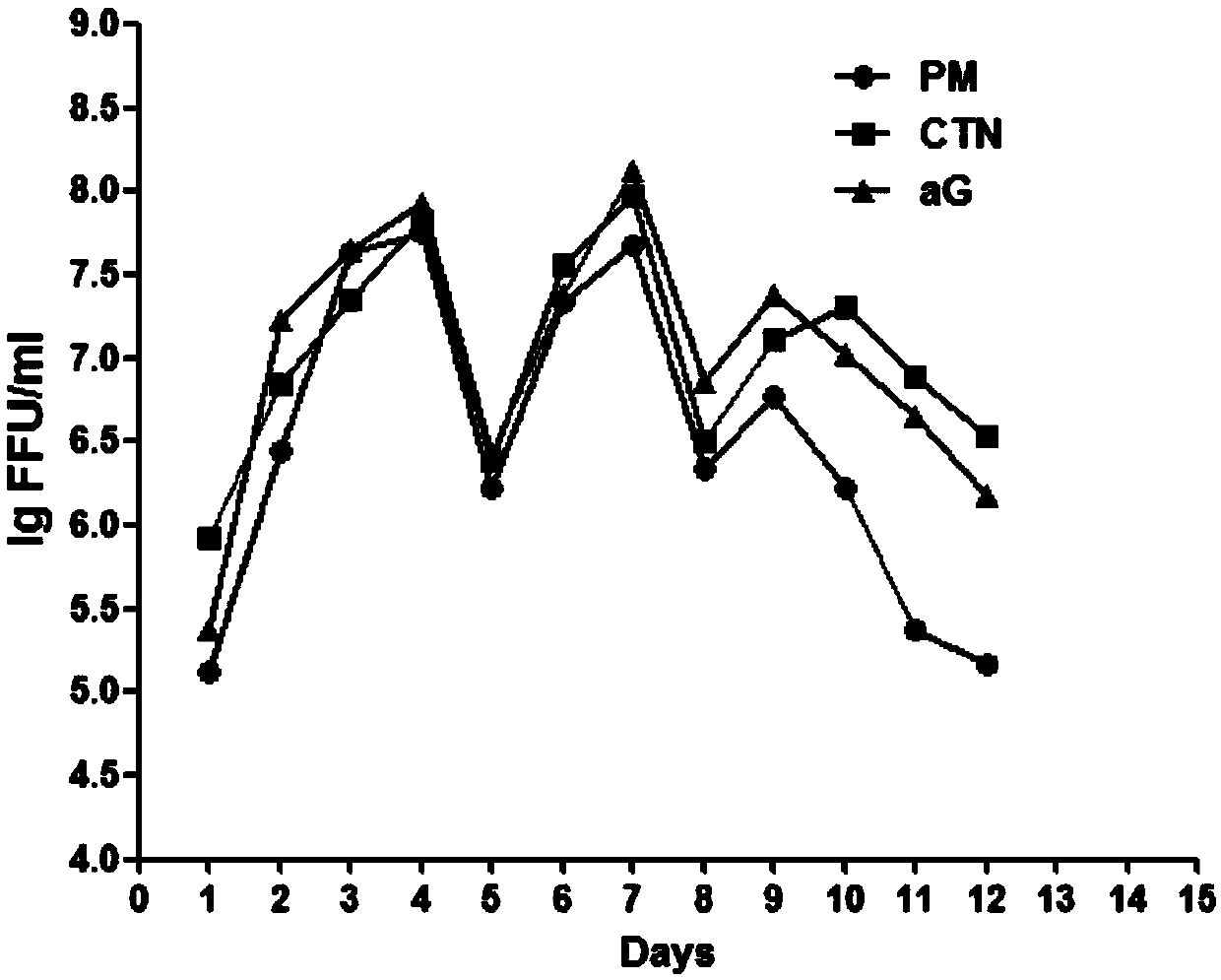
[0045] Embodiment 1PM strain rabies virus is respectively subcultured in MRC-5, 2BS cells
[0046] In this example, the PM strain of rabies virus was subcultured in human embryonic lung diploid cells (MRC-5) and human diploid cells (2BS) respectively.
[0047] MRC-5 and 2BS cells were revived in 1.1 and inoculated on T-25cm 2 In a plastic culture flask, after the cells grow into a monolayer, wash with PBS, digest into single cells with 0.25% trypsin, pass down to 25-35 passages at a ratio of 1:2-1:4 for later use.
[0048] 1.2 Inoculate the rabies virus PM strain described in step 1.1 into MRC-5 cells growing into a dense monolayer at an ultra-high MOI of 1000. After infection and adsorption for 0.25-2 hours, discard the virus inoculum and wash with PBS; after washing, add 1 2% fetal bovine serum MEM culture medium containing 1% trehalose, cultured for 2-4 days, then discarded; adding 2% fetal bovine serum MEM culture medium containing 1% trehalose, cultured for 4-6 days to h...
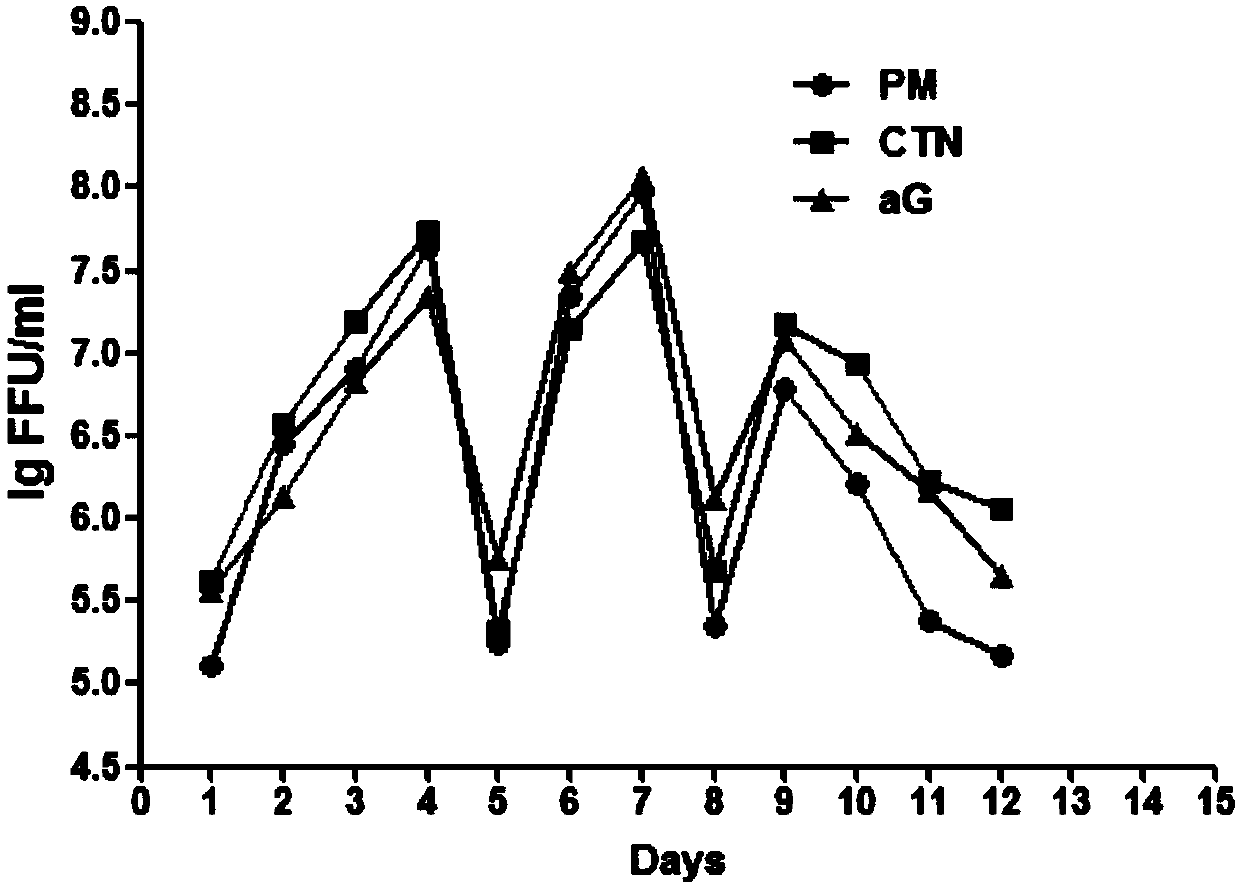
Embodiment 2
[0060] Example 2 CTN strain virus was subcultured in MRC-5 and 2BS cells respectively
[0061] Using the method for passage of virus seeds in Example 1, the CTN strain virus was screened and passaged on MRC-5 and 2BS cells, and the virus titer was detected by direct immunofluorescence method. The results are shown in Table 2.
[0062] Table 2
[0063]
[0064] The results show that when the CTN strain virus is passed to the 6th generation, the virus titer can reach more than 7.5lgFFU / ml, which is 7.0logLD compared with the existing technology when it is passed to the P15th generation 50 / ml method, the present invention greatly shortens the virus adaptation period, and the virus titer is higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com