Method for directly preparing acellular hypertrophic cartilage matrix from adipose tissue
A technology of adipose tissue and cartilage matrix, applied in the direction of bone/connective tissue cells, animal cells, tissue regeneration, etc., can solve the problems of low osteogenesis efficiency and low osteoinductive performance, and achieve high differentiation potential and reduced immunogenicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
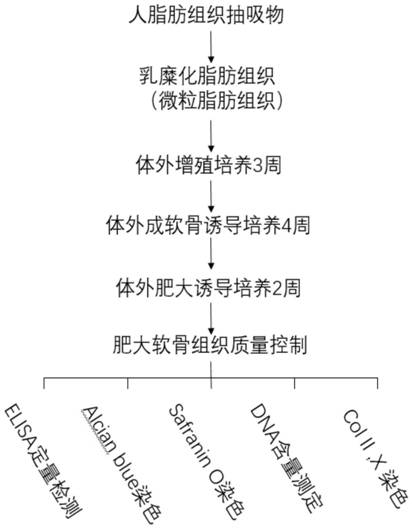
[0045] A method for directly preparing acellular hypertrophic cartilage matrix from adipose tissue, comprising the following steps:
[0046] (1) Preparation of particulate adipose tissue;
[0047] The preparation of particulate adipose tissue is a mature technique in the art, and the steps in this example are as follows:
[0048] (a) Human adipose tissue obtained from liposuction operation, after repeated rinsing with saline, the upper layer of adipose tissue was collected;
[0049] (b) Cut the upper layer of fat tissue with scissors, centrifuge at 1000-2000g for 3-5min, remove the upper layer of fat and the lower part of swelling fluid, and collect the middle fat layer in a 20mL syringe;
[0050] (c) Use a three-way tube to connect two 20mL syringes, inject the two syringes back and forth 30 times, and obtain particulate adipose tissue;
[0051] (d) The particulate adipose tissue is centrifuged at 1000-2000 g for 3-5 minutes to remove the upper layer of fat layer, and the l...
Embodiment 2
[0077] The culture medium used in this embodiment consists of the following:
[0078] Serum-free basal medium (SFM):
[0079] DMEM culture fluid + 1% HSA + 1% PSG + 1% HEPES + 2% ITS + 1.2% linoleic acid;
[0080] Hypertrophy Inducing Medium:
[0081] SFM+β-glycerophosphate disodium salt (10 -2 mol / L)+dexamethasone (10 -8 mol / L)+ascorbic acid (10 -5 mol / L).
[0082] Chondrogenic induction medium: SFM+dexamethasone (10 -7 mol / L)+ascorbic acid (10 -5 mol / L)+BMP-6(20ng / mL)+TGF-β 3 (5ng / mL);
[0083] Applying the above medium to the method in Example 1 also meets the requirements.
Embodiment 3
[0085] The culture medium used in this embodiment consists of the following:
[0086] Chondrogenic induction medium:
[0087] SFM+BMP-6(5ng / mL)+TGF-β 3 (20ng / mL)+dexamethasone (10 -7 mol / L)+ascorbic acid (10 -5 mol / L);
[0088] Hypertrophy Inducing Medium:
[0089] SFM+β-glycerophosphate disodium salt (10 -2 mol / L)+dexamethasone (10 -8 mol / L)+ascorbic acid (10 -5 mol / L).
[0090] Serum-free basal medium (SFM):
[0091] DMEM culture fluid+1%HSA+1%PSG+1%HEPES+0.5%ITS+0.3%linoleic acid;
[0092] Applying the above medium to the method in Example 1 also meets the requirements.
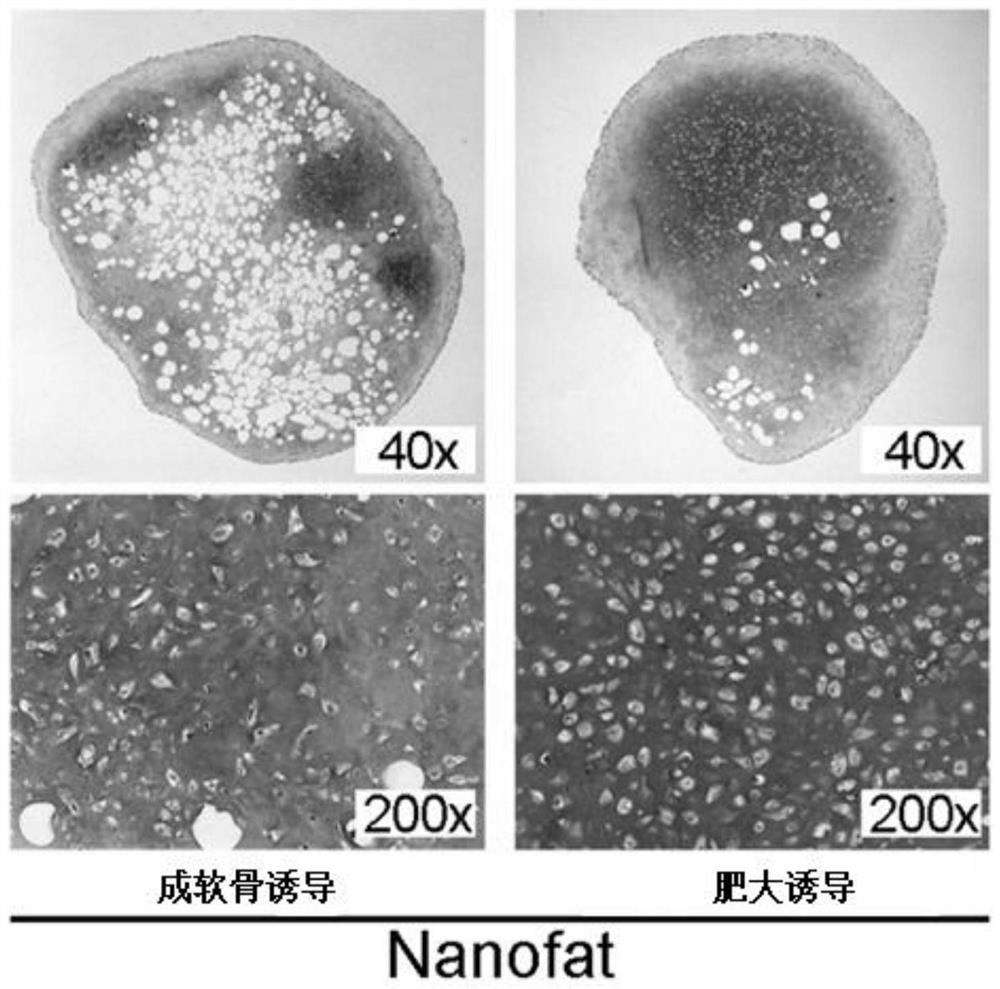
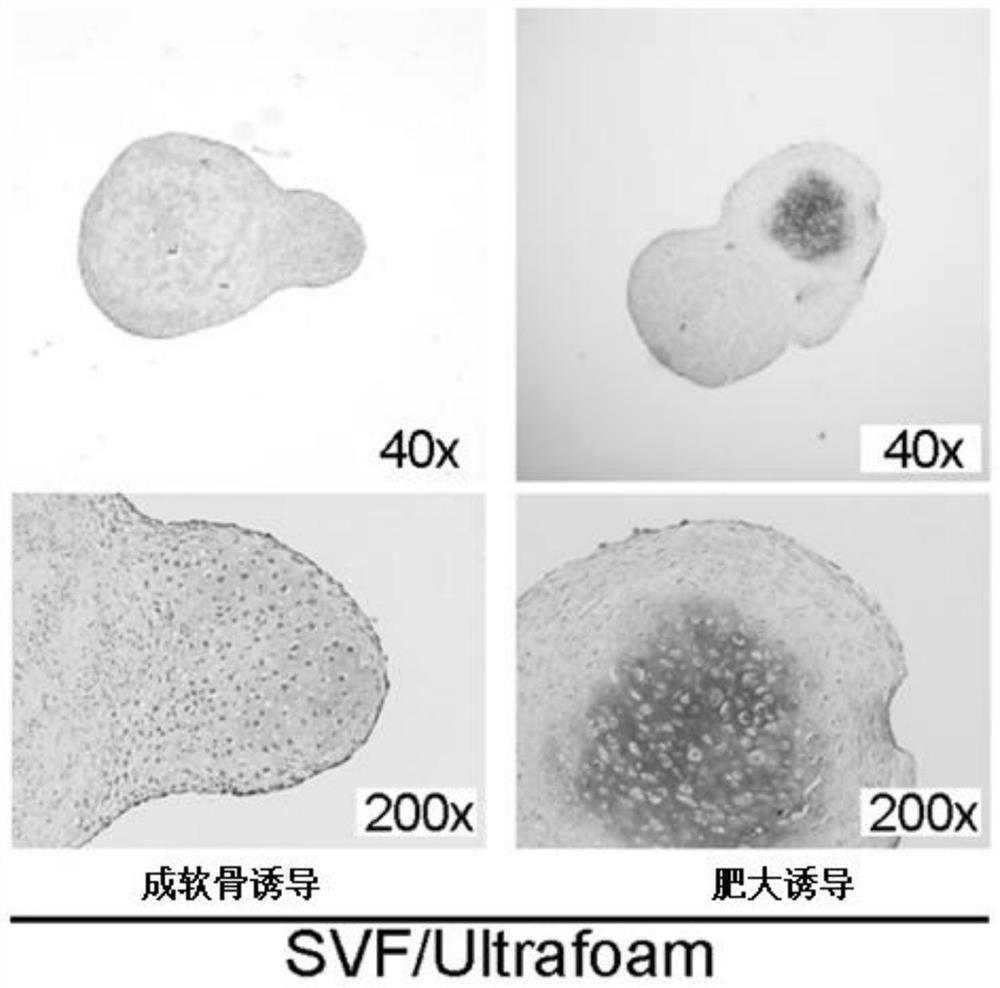
[0093] Our study found that ASC in adipose tissue can exist in the form of stem cell niche under the protection of extracellular matrix (ECM), and ASC in its protection can form endochondral bone , directly induce differentiation into hypertrophic cartilage tissue. Moreover, the hypertrophic cartilage tissue can form ectopic bone even under the skin of nude rats, regenerating bone tissue rich i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com