Non-covalent polymer catalyst suitable for oxidization of 2,3,6-trimethylphenol and preparation method of non-covalent polymer catalyst
A technology of trimethylphenol and catalyst, which is applied in the field of non-covalent polymer catalysts and its preparation for the oxidation of 2,3,6-trimethylphenol, which can solve the problems of separation of products and catalysts, environmental pollution, and stability of small molecules Poor performance and other problems, to achieve the effect of good industrial application prospects, simple preparation method, and product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The invention provides a method for preparing a non-covalent polymer catalyst suitable for oxidation of 2,3,6-trimethylphenol, comprising the following steps:
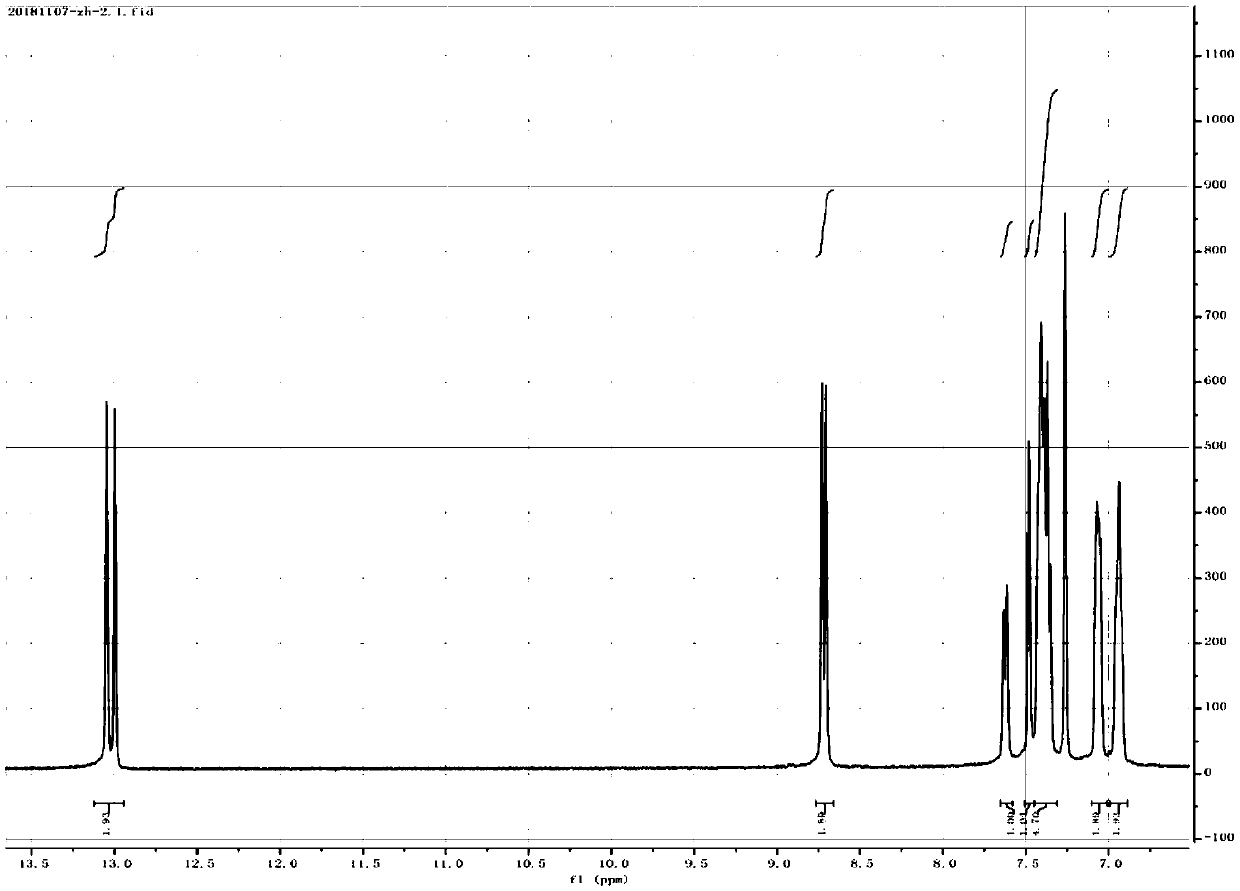
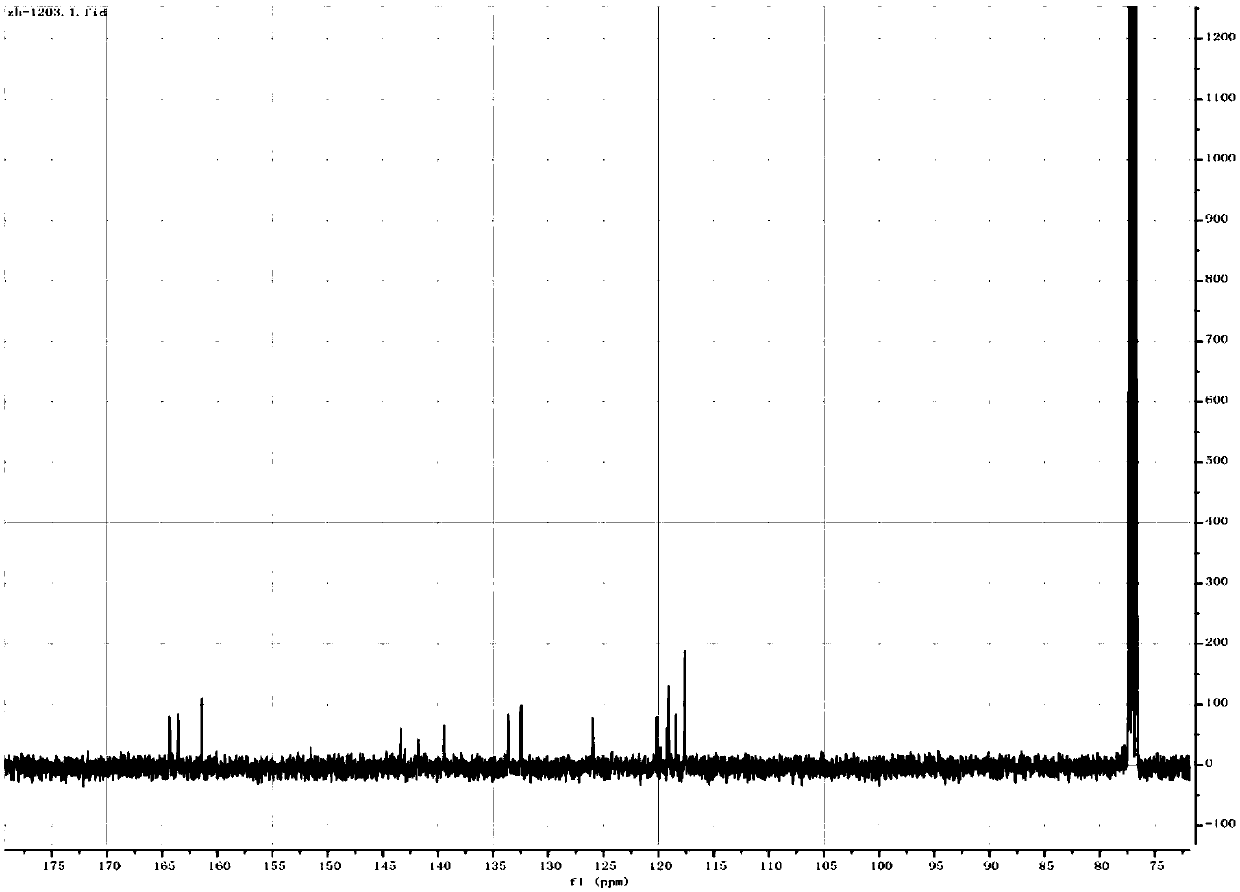
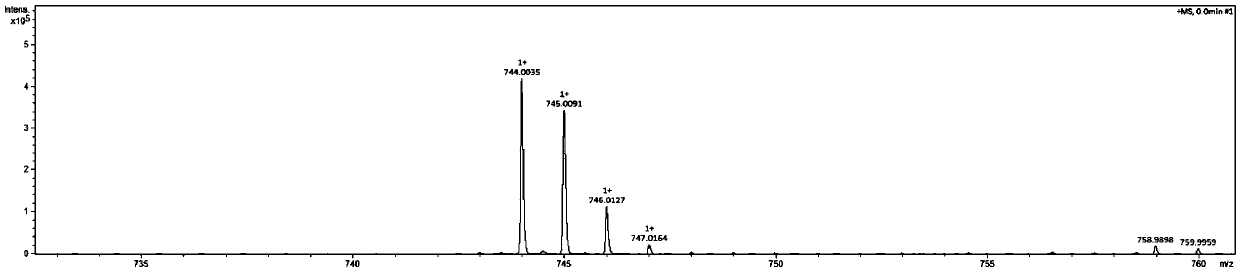
[0042] (1) Synthesis of Salphen: In a 500ml three-necked flask, add 3,3-diaminobenzidine (1g, 4.67mmol) and 150ml of methanol, under nitrogen protection, dissolve salicylaldehyde (2.74g, 22.4mmol) in 100ml of methanol dropwise Added to the reaction solution, stirred at room temperature for 24h, filtered, washed with methanol to obtain the crude product, recrystallized from THF to obtain the desired Salphen compound, wherein the bisalphen structure is as follows:
[0043]
[0044] (2) Synthesis of cobalt (Salphen): In a 250ml three-necked flask, add Salphen (251mg, 0.4mmol) and 100ml THF, ultrasonically dissolve, under nitrogen protection, methanol (30ml) of cobalt acetate tetrahydrate (238mg, 0.95mmol) The solution was added dropwise to the THF solution of bi-Salphen, stirred at room temperature for 24 h, fil...
Embodiment 2
[0049] The invention provides a method for preparing a non-covalent polymer catalyst suitable for oxidation of 2,3,6-trimethylphenol, comprising the following steps:
[0050] (1) Synthesis of Salphen: In a 500ml three-necked flask, add 3,3-diaminobenzidine (1g, 4.67mmol) and 150ml of methanol, under nitrogen protection, dissolve salicylaldehyde (2.74g, 22.4mmol) in 100ml of methanol dropwise Added to the reaction solution, stirred at room temperature for 24h, filtered and washed with methanol to obtain the crude product, THF recrystallized to obtain the desired Salphen compound;
[0051] (2) Synthesis of cobalt (Salphen): In a 250ml three-necked flask, add Salphen (251mg, 0.4mmol) and 100ml THF, ultrasonically dissolve, under nitrogen protection, methanol (30ml) of cobalt acetate tetrahydrate (238mg, 0.95mmol) The solution was added dropwise to the THF solution of Salphen, stirred at room temperature for 24h, filtered, washed with methanol and water to obtain the desired Co(Sa...
Embodiment 3
[0055] The invention provides a method for preparing a non-covalent polymer catalyst suitable for oxidation of 2,3,6-trimethylphenol, comprising the following steps:
[0056] (1) Synthesis of Salphen: In a 500ml three-necked flask, add 3,3-diaminobenzidine (1g, 4.67mmol) and 150ml of methanol, under nitrogen protection, dissolve salicylaldehyde (2.74g, 22.4mmol) in 100ml of methanol dropwise Added to the reaction solution, stirred at room temperature for 24h, filtered and washed with methanol to obtain the crude product, THF recrystallized to obtain the desired Salphen compound;
[0057] (2) Synthesis of cobalt (Salphen): In a 250ml three-necked flask, add Salphen (251mg, 0.4mmol) and 100ml THF, ultrasonically dissolve, under nitrogen protection, methanol (30ml) of cobalt acetate tetrahydrate (238mg, 0.95mmol) The solution was added dropwise to the THF solution of Salphen, stirred at room temperature for 24h, filtered, washed with methanol and water to obtain the desired Co(Sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com