A method for inhibiting galvanic corrosion of molten salt system
A galvanic corrosion and system technology, applied in the field of inhibiting galvanic corrosion in molten salt systems, can solve problems such as the difficulty of realizing the protective layer, and achieve the effect of reducing the potential difference and inhibiting galvanic corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
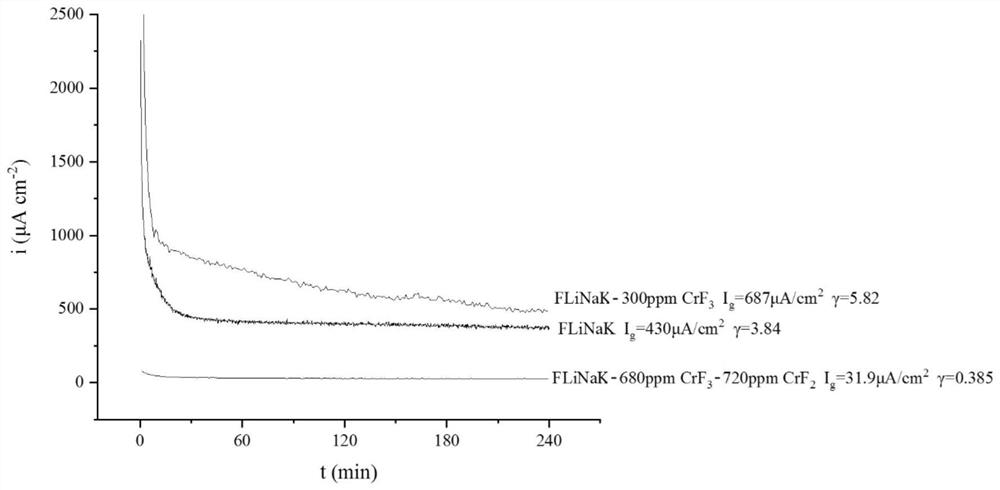
[0050] Add 680ppmCrF to FLiNaK molten salt system 3 and 720ppmCrF 2 , recorded as FLiNaK-680ppmCrF 3 -720ppmCrF 2 .
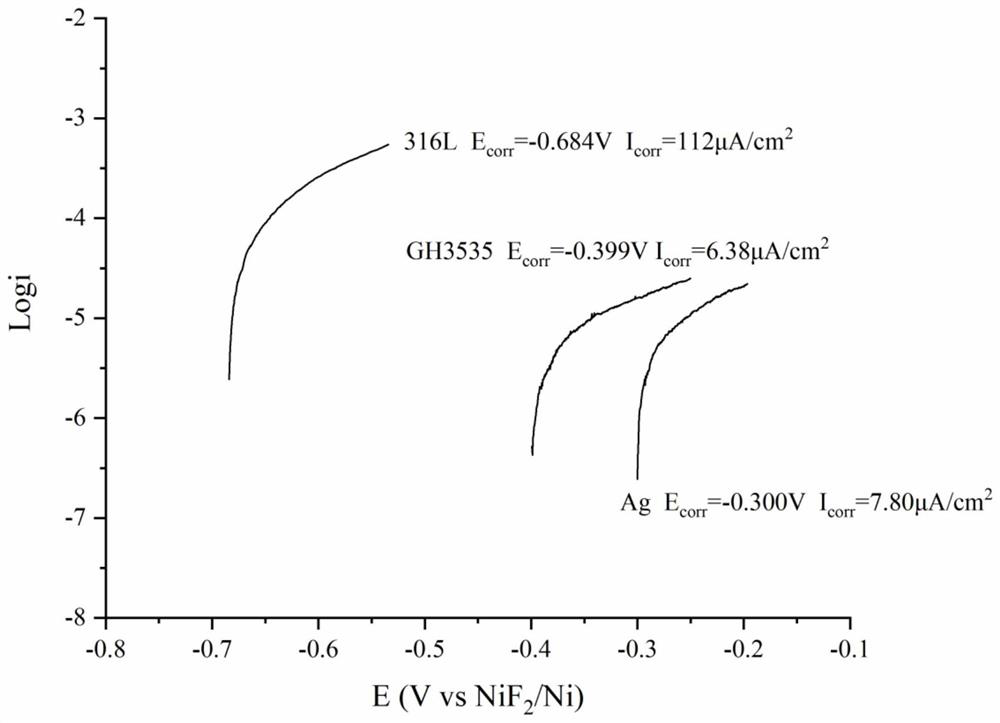
[0051] Using the above method to make NiF 2 / Ni is the reference electrode, and graphite is the counter electrode. 316L stainless steel, GH3535 nickel-based alloy and silver were tested at 600°C in FLiNaK-680ppmCrF 3 -720ppmCrF 2 The anodic polarization curve in (where silver is used as the working electrode for comparison), such as Figure 7 shown. It can be seen from the figure that the self-corrosion potentials of the three metals in the molten salt system tend to be the same, about -0.724±0.001V, and the potential difference is reduced to within a few millivolts, which can greatly reduce the occurrence of galvanic corrosion between different metals. possible.
[0052] Using the above method also tested 316L stainless steel and GH3535 nickel-based alloy at 600 °C in FLiNaK-680ppmCrF 3 -720ppmCrF 2 Galvanic current in molten salt, so as to obtain 31...
Embodiment 2
[0055] To further elucidate the formation of redox buffer molten salts and their effect on the self-corrosion potential of different metallic materials,
[0056] In this example, 1000ppmCrF is added to the FLiNaK molten salt system 3 , recorded as FLiNaK-1000ppmCrF 3 , in the FLiNaK-1000ppmCrF 3 Add 50ppmCrF to 2 , 100ppmCrF 2 , 400ppmCrF 2 , 600ppmCrF 2 , 800ppmCrF 2 , 1000ppmCrF 2 , 1200ppmCrF 2 and 1500ppmCrF 2 , respectively recorded as FLiNaK-1000ppmCrF 3 -50ppmCrF 2 , FLiNaK-1000ppmCrF 3 -100ppmCrF 2 , FLiNaK-1000ppmCrF 3 -400ppmCrF 2 , FLiNaK-1000ppmCrF 3 -600ppmCrF 2 , FLiNaK-1000ppmCrF 3 -800ppmCrF 2 , FLiNaK-1000ppmCrF 3 -1000ppmCrF 2 , FLiNaK-1000ppmCrF 3 -1200ppmCrF 2 and FLiNaK-1000ppmCrF 3 -1500ppmCrF 2 .
[0057] Using the above method to NiF 2 / Ni is the reference electrode, and graphite is the counter electrode. The anodic polarization curves of 316L stainless steel, GH3535 nickel-based alloy and silver in the above molten salt system...
Embodiment 3
[0059] In order to illustrate that the method of the present invention is applicable to the suppression of galvanic corrosion between various molten salts and various materials, different redox ion pairs were added to different molten salts in this example, and the above method was also used to test The galvanic corrosion effect coefficients of different materials in these systems are shown in Table 1.
[0060] Table 1
[0061]
[0062] Among the molten salts in Table 1, 46.5LiF-11.5NaF-42KF means that the molten salt is a mixture of LiF, NaF and KF, wherein the molar ratio of LiF, NaF and KF is 46.5:11.5:42. 66.7LiF-33.3BeF 2 Indicates that the molten salt is a mixture of LiF and BeF, wherein the molar ratio of LiF and BeF is 66.7:33.3.33NaCl-21.6KCl-45.4MgCl 2 Indicates that the molten salt is NaCl, KCl and MgCl 2 A mixture of which, NaCl, KCl and MgCl 2 The molar ratio is 33:21.6:45.4. 33NaCl-21.6KCl-45.4CaCl 2 Indicates that the molten salt is NaCl, KCl and MgCl 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com