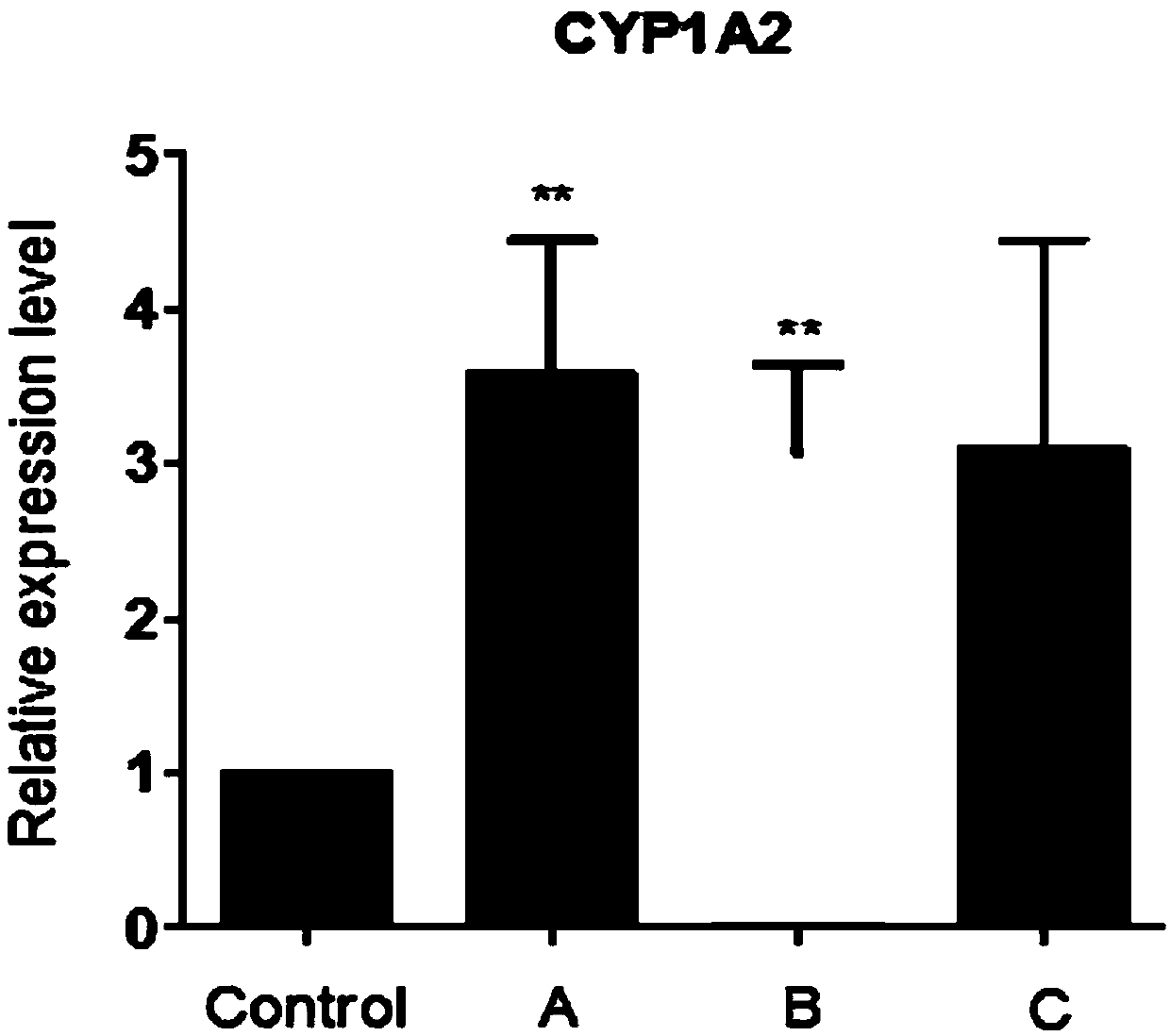
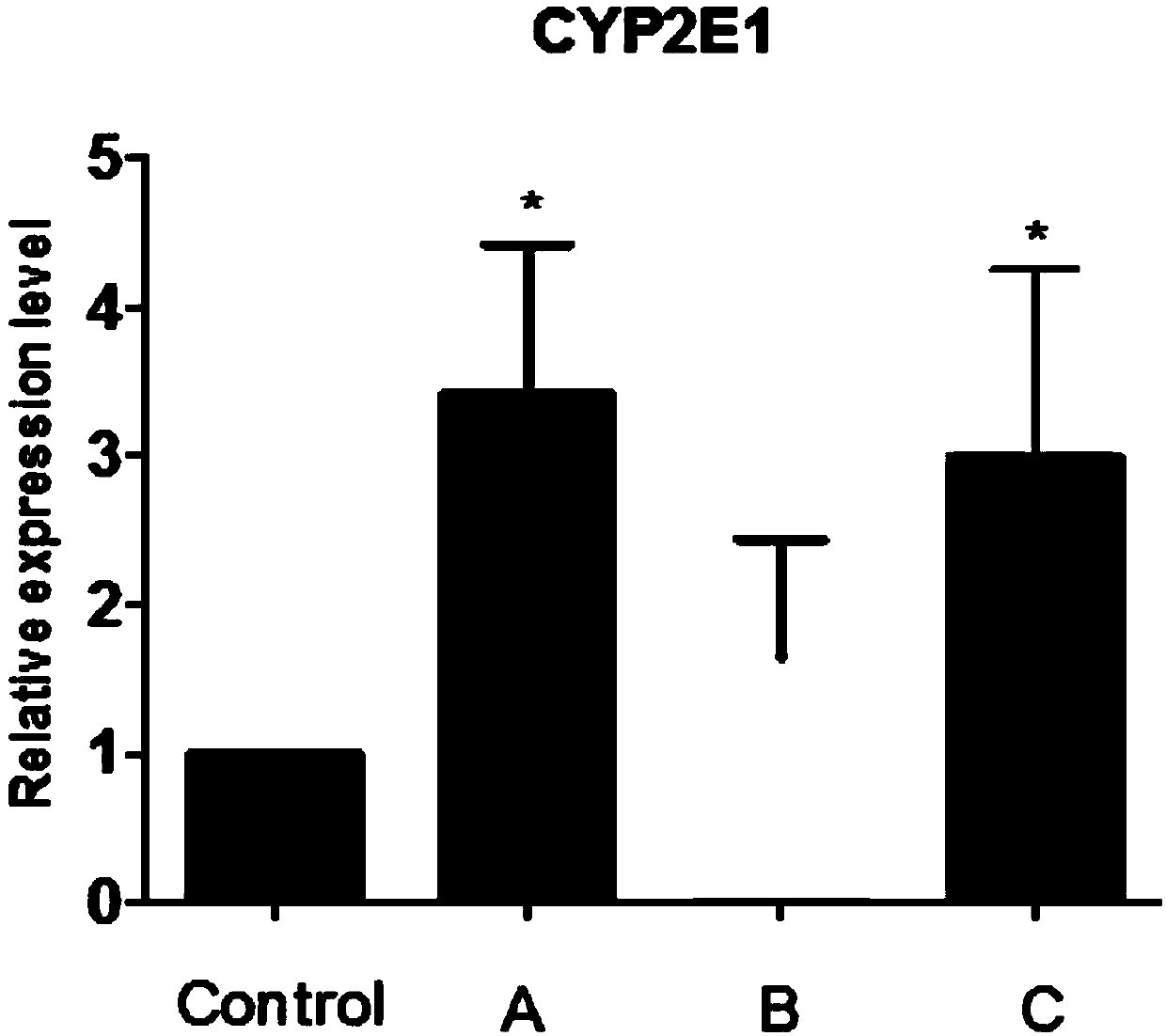
Complete culture medium for culturing hepatocytes
A technology of complete medium and hepatocytes, which is applied in the cultivation process, animal cells, tissue culture, etc., can solve the problems of discounting the effect of cytology experiments, and achieve the effects of avoiding ethical risks, saving costs, reducing time consumption and costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] One, the preparation of described complete culture medium:
[0052] Only the Hyclone DMEM medium formula is selected as the basal medium in the examples under the specific implementation mode, and other basal mediums can be selected under the premise that other experiments or culture conditions permit and do not deviate from the idea of the present invention. Described Hyclone DMEM medium formula is as follows:
[0053] Inorganic salts include at least:
[0054]
[0055] Amino acids include at least:
[0056]
[0057] Vitamins include at least:
[0058]
[0059]
[0060] Other substances are at least:
[0061] D-glucose 4500mg / L
[0062] Sodium phenol red 15.9mg / L
[0063] Sodium bicarbonate 3700mg / L
Embodiment 1
[0064] Embodiment 1: the preparation of complete medium 1:
[0065] In the present embodiment, the powder of the above-mentioned Hyclone DMEM medium formula is used as the base medium and the complete medium of the present invention is configured on this basis. The specific steps are as follows:
[0066] 1) Disinfect or sterilize the equipment and utensils used for configuration, including but not limited to serum bottles, magnetic stirring beads, volumetric flasks, beakers, pH meters, etc.;
[0067] 2) collecting the portal vein blood of fresh Tibetan minipigs;
[0068] 3) Centrifuge the portal vein blood with a centrifugal force of 3000×g for 12 minutes, and remove the supernatant;
[0069] 4) heating the lower supernatant at 56° C. to inactivate the complement to obtain the inactivated supernatant;
[0070] 5) Filter the inactivated supernatant through a microporous membrane with a filter hole diameter of 0.22 μm, collect porcine portal vein serum, and store it for future...
Embodiment 2
[0079] Embodiment 2: the preparation of complete medium 2:
[0080] In the present embodiment, the powder of the above-mentioned Hyclone DMEM medium formula is used as the base medium and the complete medium of the present invention is configured on this basis. The specific steps are as follows:
[0081] 1) Disinfect or sterilize the equipment and utensils used for configuration, including but not limited to serum bottles, magnetic stirring beads, volumetric flasks, beakers, pH meters, etc.;
[0082] 2) collecting the portal vein blood of fresh Tibetan minipigs;
[0083] 3) Centrifuge the portal vein blood with a centrifugal force of 3000×g for 12 minutes, and remove the supernatant;
[0084] 4) heating the lower supernatant at 56° C. to inactivate the complement to obtain the inactivated supernatant;
[0085] 5) Filter the inactivated supernatant through a microporous membrane with a filter hole diameter of 0.22 μm, collect porcine portal vein serum, and store it for future...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com