Method for preparing parecoxib freeze-dried powder injection
A technology for freeze-dried powder injection and parecoxib, which is applied in the field of preparation of parecoxib freeze-dried powder for injection, can solve problems such as manual operation and increase drying cost, achieve convenient inflow and outflow, and save drying energy consumption , Improve the cleaning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A method for preparing parecoxib freeze-dried powder injection, comprising:
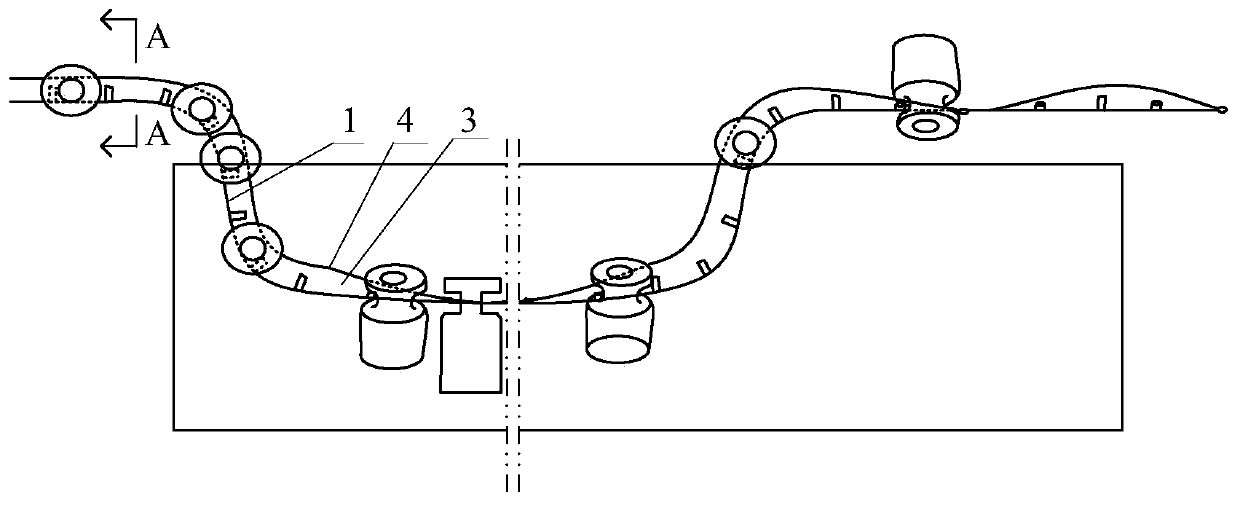
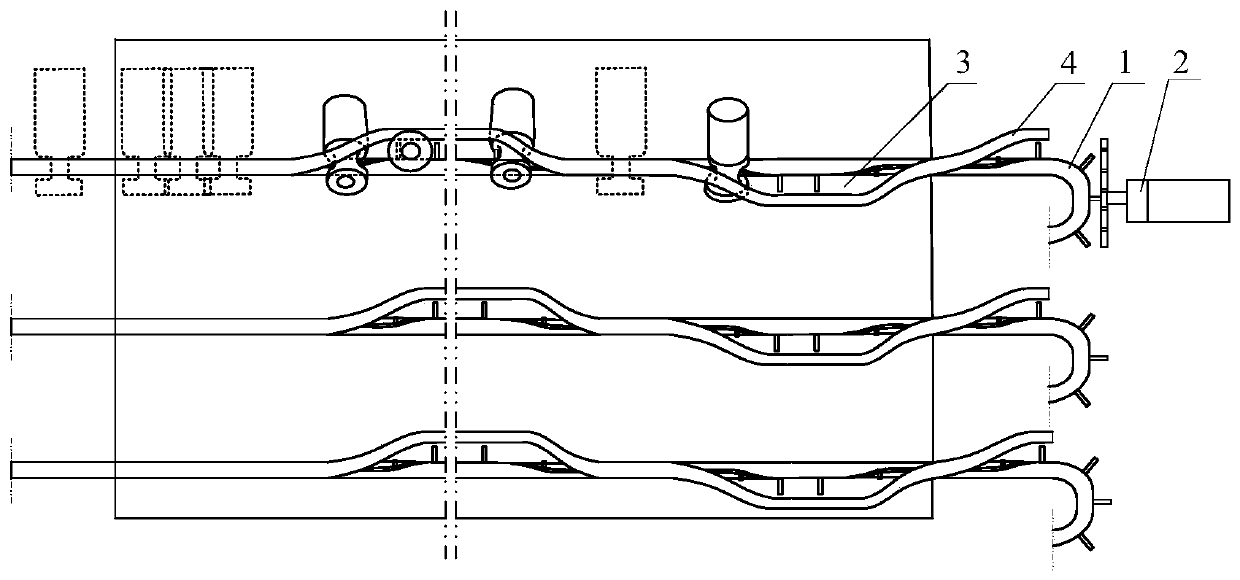
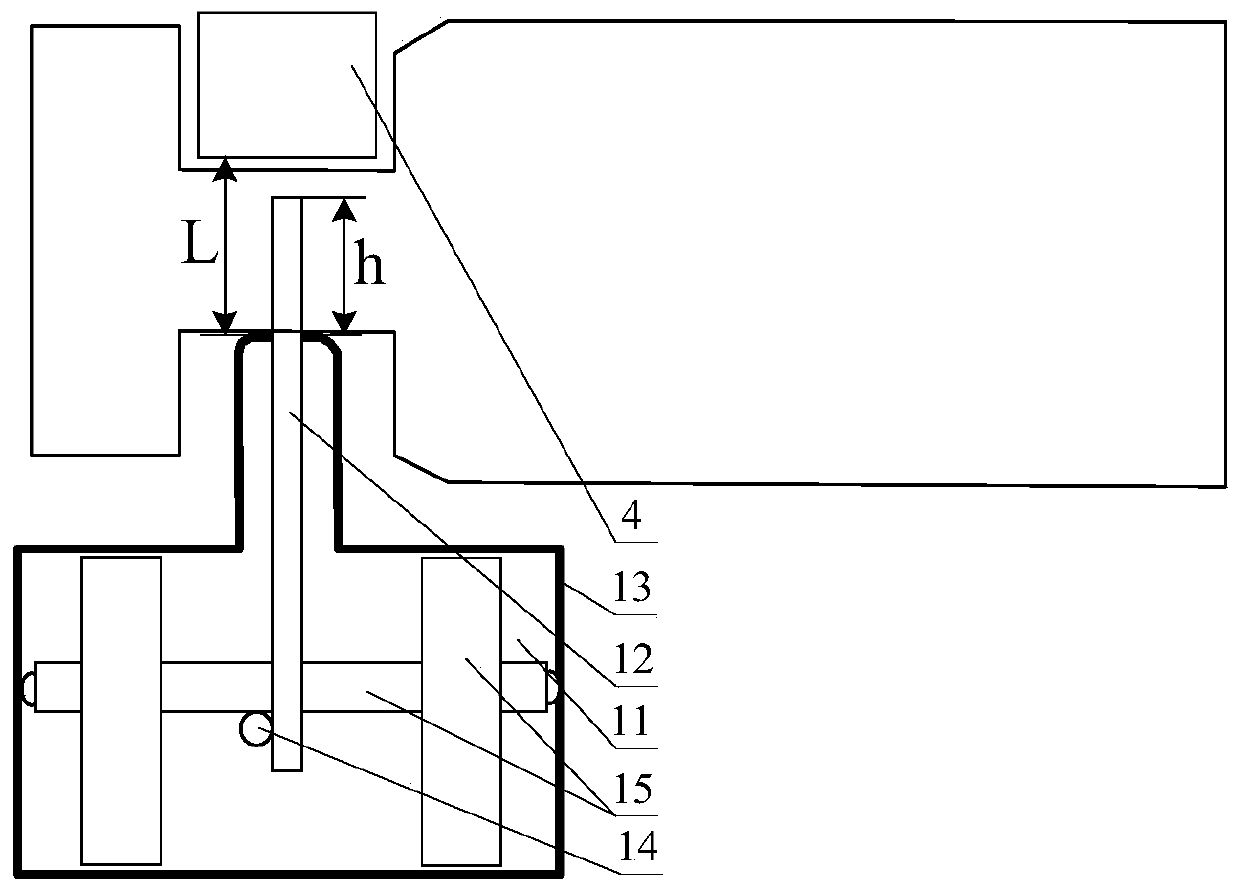
[0043] Step 1. Place the vials on the transmission line, and transport the vials to the cleaning tank through the transmission line for cleaning; the transmission line includes a transmission belt 1, a driving device 2 that drives the transmission belt 1 to rotate, and is arranged on the top of the transmission belt 1 and the transmission The restriction strip 4 of the bottleneck passage 3 of the vial is formed between the belts 1 . The conveyor belt 1 includes an in-plane moving section for transporting the vials according to the transport direction, and a rotating section for rotating the vials by 30 degrees, 45 degrees or 90 degrees perpendicular to the transport direction. Such as Figure 4 As shown in , the opening at the top of the in-plane moving section is arranged along the conveying direction. Such as Figure 5 As shown, the opening at the top of the rotating segment spirally surr...
Embodiment 2
[0055] The difference between this embodiment and Embodiment 1 is that the structure of the present invention is further optimized in this embodiment, and the specific settings are as follows:
[0056] The length of the rotating segment is greater than 5 cm. The vials transported in the in-plane moving section are arranged parallel to the horizontal plane. The flexible connecting strip 14 is a chain or a nylon rope. The driving device 2 includes a drive gear that drives the flexible connecting strip 14 to move by driving the push rod 12, and a drive motor that drives the drive gear to move; the drive gear includes an endless belt, and the meshing teeth arranged on the inner wall of the endless belt, The driving teeth arranged on the outer wall of the endless belt, and the rotating wheel arranged in the endless belt and meshed with the meshing teeth, the driving motor drives the rotating wheel to rotate and then drives the endless belt to rotate, and the rotation of the endles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com